Boston Scientific accounts are for healthcare professionals only.






The Vertiflex™ Procedure† Superion™ Indirect Decompression System
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Ordering information
- Training
- Resources
Setting the standard of care for lumbar spinal stenosis
The Vertiflex Procedure is redefining the treatment of LSS for patients. This Level 1 evidence-based procedure is supported by data from patients who reported successful outcomes up to five years.
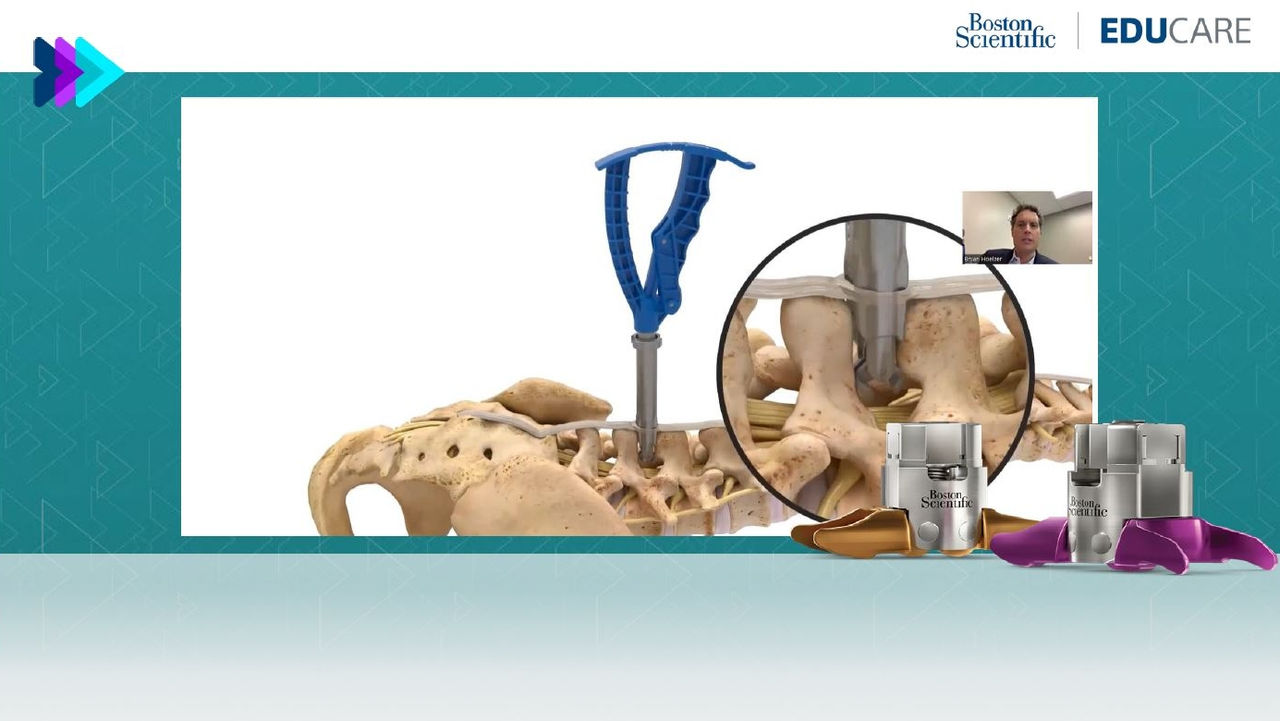
How it works
The Vertiflex Procedure treats the root cause of pain by lifting pressure from the nerve roots at the affected segment.
Why choose the Vertiflex Procedure
For patients with moderate LSS, the Vertiflex Procedure is designed to provide clinically proven, long-term relief.
Key features:
- Allows for indirect decompression of the nerves while preserving the sagittal balance of the spine
- Backed by 5-year Level 1 Randomized Control Trial (RCT) and real-world data1-5
- Only standalone interspinous spacer FDA approved to treat moderate LSS
- Clinically proven to treat all forms of moderate LSS: central, lateral and foraminal
- As a reversible procedure, all future surgical options remain open
Subscribe for updates on the Vertiflex Procedure
Receive timely access to major announcements, opportunities to connect with your peers through educational events, and useful tools for you to help more patients.
† The Superion Indirect Decompression System (IDS)
§ At 12 months and from the population that completed the follow-up
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
Results from clinical studies are not predictive of results in other studies. Results in other studies may vary.
References:
- Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, MilPatel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Davis RP, Miller LE, Block JE, Geisler FH. Superion interspinous process spacer for intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis: two-year results from a randomized controlled FDA-IDE pivotal trial. Spine (Phila Pa 1976). 2015 Mar 1;40(5):275-82.
- Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Miller LE, Block JE, Geisler FH. Two-year clinical outcomes of a multicenter randomized controlled trial comparing two interspinous spacers for treatment of moderate lumbar spinal stenosis. BMC Musculoskelet Disord. 2014 Jul 5;15:221.
- Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017;12:1409-1417. (N = 88)
- Nunley PD, Deer TR, Benyamin RM, Staats PS, Block JE. Interspinous process decompression is associated with a reduction in opioid analgesia in patients with lumbar spinal stenosis. J Pain Res. 2018;11:2943-2948. (N = 107)
- Whang P, Antony A, Chen L, et al. Biomechanical Impact of Interspinous Spacers on Sagittal Alignment in Patients with Lumbar Spinal Stenosis [Abstract]. Twenty-Fifth Annual Meeting of the North American Neuromodulation Society, January 13-15, 2022, Orlando, FL USA.