Save time & improve outcomes1,2
It’s time for a new standard in DBS.
Boston Scientific accounts are for healthcare professionals only.
Create an account to access online training and education on EDUCARE, manage your customer profile, and connect with customer support and service teams.
My Boston Scientific account
Access your online applications and manage your customer profile.
Quick Links
Call customer care
It’s time for a new standard in DBS.
Clinicians share their experience with Image Guided Programming
Learn how Image Guided Programming is impacting the efficiency of patient programming
Illumina 3D Technology enables easy and efficient deep brain stimulation (DBS) programming by automatically generating a patient-specific stimulation plan in 3 easy steps.
Select target anatomy & avoid region(s)

Algorithm generates a patient-specific stimulation plan

Illumina 3D steering enables easy adjustments of stimulation

56% reduction
in programming time1
20 minutes
average programming time1
Improved outcomes
84% of patients experienced lasting motor and quality of life improvements after being re-programmed using image guided programming (n=31)2
Through our exclusive partnership with Brainlab, DBS programmers can use the Boston Scientific Clinician Programmer with Brainlab Elements to identify the location of the leads within the patient's specific anatomy to inform programming.
Brainlab Elements are efficient, effective, a la carte software modules serving multiple clinical specialties.
With Brainlab Elements Lead Localization and Elements Anatomical Mapping, neurosurgeons and neurologists can see the Vercise™ Cartesia™ Directional Lead in the patient’s own segmented anatomy and correlate stimulation location to outcomes to assist in programming.
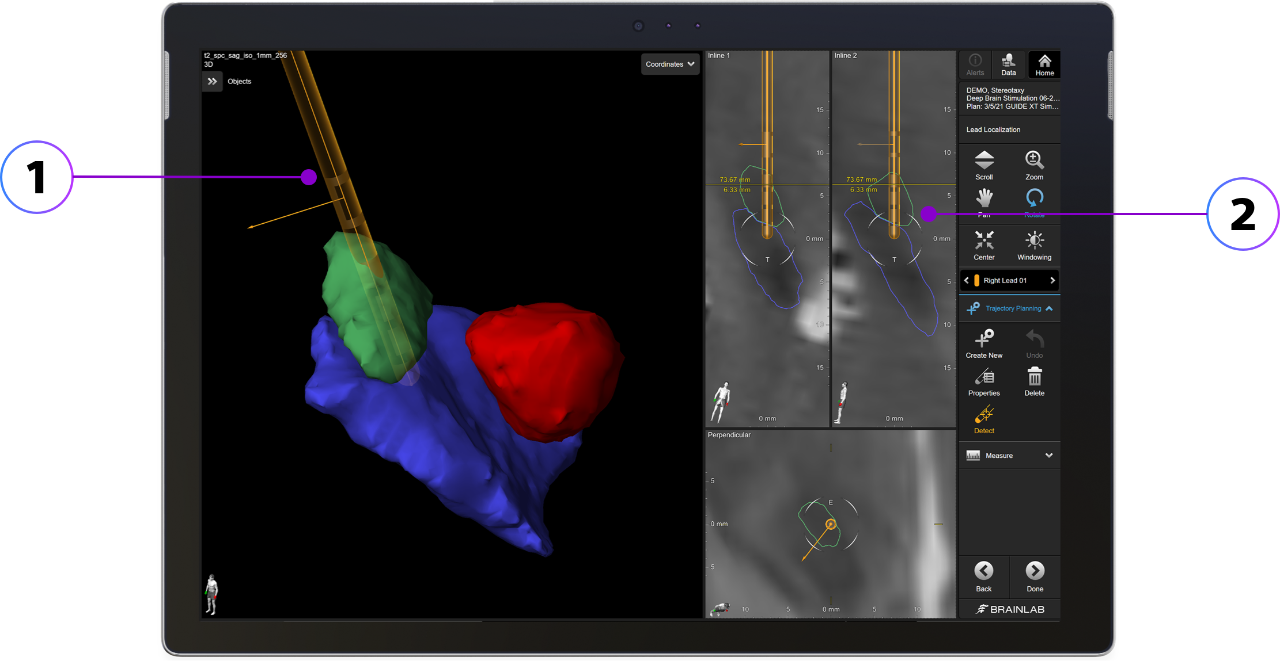
Image provided by Brainlab
1. Display of lead orientation within the anatomy
2. Identify contacts within the target
With Brainlab Elements you are able to:
This offers an informed starting point for DBS programming and reduces programming time.
Easy and efficient documentation with EMR connectivity
If you are a patient looking for more information on DBS, visit DBSandMe.com.
Reference:
1. Image Guided programming in PD patients enables a reduction in programming time compared with standard clinical based programming (p=39).
Lange F, Et al. Reduced Programming Time and Strong Symptom Control Even in Chronic Course Through Imaging-Based DBS Programming. Front Neurol. 2021 Nov 8;12:785529. N=10
2. Torres V, et al. Image-guided programming deep brain stimulation improves clinical outcomes in patients with Parkinson’s disease. NPJ Parkinsons Dis. 2024 Jan 27;10(1):29.
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
Brainlab Elements Image Fusion is an application for the co-registration of image data within medical procedures by using rigid and deformable registration methods. It is intended to align anatomical structures between data sets. The device itself does not have clinical indications.
The Brainlab Elements Trajectory Planning software is intended for pre-, intra- and postoperative image-based planning and review of either open or minimally invasive neurosurgical and neurological procedures. Its use is indicated for any medical condition in which the use of stereotactic surgery may be appropriate for the placement of instruments/devices and where the position of the instrument / device can be identified relative to images of the anatomy. This includes, but is not limited to, the following Cranial procedures (including frame-based stereotaxy and frame alternative-based stereotaxy):
iPlan's indications for use are the viewing, presentation, and documentation of medical imaging, including different modules for image processing, image fusion, atlas assisted visualization and segmentation, intraoperative functional planning where the output can be used e.g. with stereotactic image guided surgery or other devices for further processing and visualization. Example procedures include, but are not limited to: Planning and simulation of cranial surgical procedures such as tumor resection, shunt placement, minimal-invasive stereotactic interventions, biopsy, planning, and simulation of trajectories for stimulation and electrode recording.
Indication for Use: The Boston Scientific Vercise™ PC, Vercise Gevia™, Vercise Genus™ Deep Brain Stimulation Systems are indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Bilateral stimulation of the internal globus pallidus (GPi) as an adjunctive therapy in reducing some of the symptoms of advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
-Unilateral thalamic stimulation of the ventral intermediate nucleus (VIM) is indicated for the suppression of tremor in the upper extremity. The system is intended for use in patients who are diagnosed with essential tremor or parkinsonian tremor not adequately controlled by medications and where the tremor constitutes a significant functional disability.
-Bilateral stimulation of the ventral intermediate nucleus (VIM) of the thalamus for the suppression of disabling upper extremity tremor in adult essential tremor patients whose tremor is not adequately controlled by medications and where the tremor constitutes a significant functional disability.
The Boston Scientific Vercise Deep Brain Stimulation System is indicated for use in:
-Bilateral stimulation of the subthalamic nucleus (STN) as an adjunctive therapy in reducing some of the symptoms of moderate to advanced levodopa-responsive Parkinson’s disease (PD) that are not adequately controlled with medication.
Contraindications, warnings, precautions, side effects: The Boston Scientific Deep Brain Stimulation (DBS) Systems or any of its components, are contraindicated for: Diathermy as either a treatment for a medical condition or as part of a surgical procedure, Electroconvulsive Therapy (ECT) and Transcranial Magnetic Stimulation (TMS) as the safety of these therapies in patients implanted with the Boston Scientific DBS System has not been established, patients who are unable to operate the system, patients who are poor surgical candidates or who experience unsuccessful test stimulation. Patients implanted with Boston Scientific DBS System without ImageReady™ MRI Technology should not be exposed to Magnetic Resonance Imaging (MRI). Patients implanted with Vercise Gevia or Vercise Genus or Vercise Genus Mixed System with M8 Adapter or Vercise DBS Lead-Only System (before Stimulator is implanted) with ImageReady MRI Technology are Full Body MR Conditional only when exposed to the MRI environment under the specific conditions defined in ImageReady MRI Guidelines for Boston Scientific DBS Systems. Assess patients for the risks of depression and suicide. This assessment should consider both the risk of depression and suicide as well as the potential clinical benefits of DBS therapy. Monitor patients for new or worsening symptoms of depression, suicidal thoughts or behaviors, or changes in mood or impulse control and manage appropriately. Refer to the Instructions for Use provided with the Boston Scientific DBS Systems or BostonScientific.com for potential adverse effects, warnings, and precautions prior to using this product. Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.