Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-center, single-arm study
First prospective study to assess outcomes after radiation segmentectomy (RS) in patients with very early or early stage hepatocellular carcinoma which showed 100% initial objective response with TheraSphere Y-90 glass microspheres.
Kim E, Sher A, Abboud G, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study [published online ahead of print, 2022 May 23]. Lancet Gastroenterol Hepatol. 2022;S2468-1253(22)00091-7. doi:10.1016/S2468-1253(22)00091-7
Overview
The aim of this study was to assess the safety and efficacy of RS in patients with unresectable hepatocellular carcinoma (HCC) deemed unfavorable for ablation. The study provides a strong rationale for new randomized trials comparing RS to ablation and supports inclusion of RS in BCLC guidelines.
Objectives
Primary endpoint: Target tumor response measured by modified RECIST (mRECIST).
Secondary endpoints: Time to progression (TTP) of the target lesion and overall disease, and adverse events using CTCAE version 5.0.
CTCAE = National Cancer Institute Common Terminology Criteria for Adverse EventsStudy design and methods
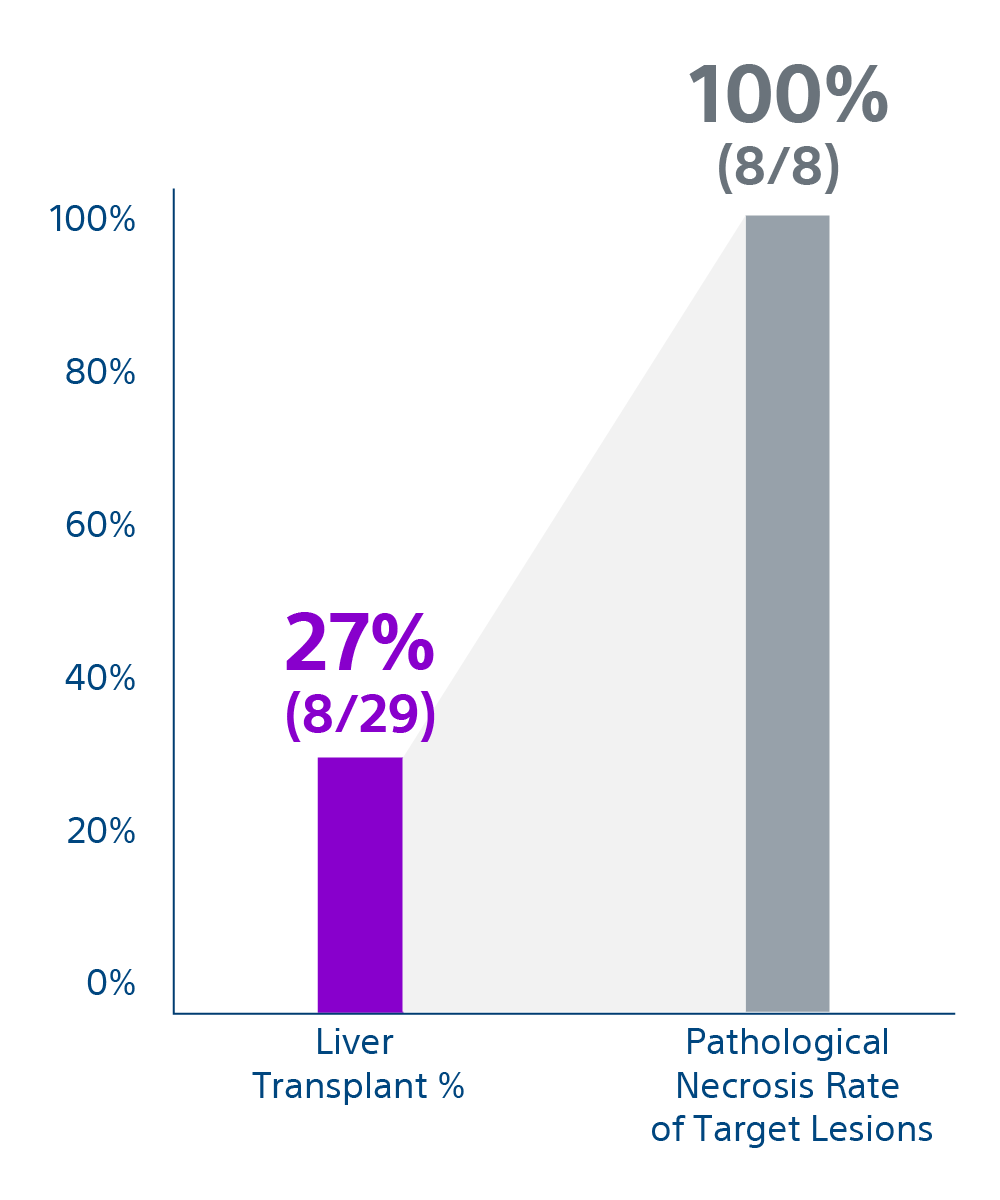
The study included adults (>18 years) with solitary HCC with unfavorable location for ablation, without metastasis or macrovascular invasion. Eligibility criteria included measurable disease ≤ 3 cm in diameter, Child-Pugh score A–B7, an ECOG score of 0, and adequate hematological and organ function. Of the 44 individuals assessed for eligibility, 29 patients were included in the study. Patients were followed up with imaging and office visits for up to 24 months.