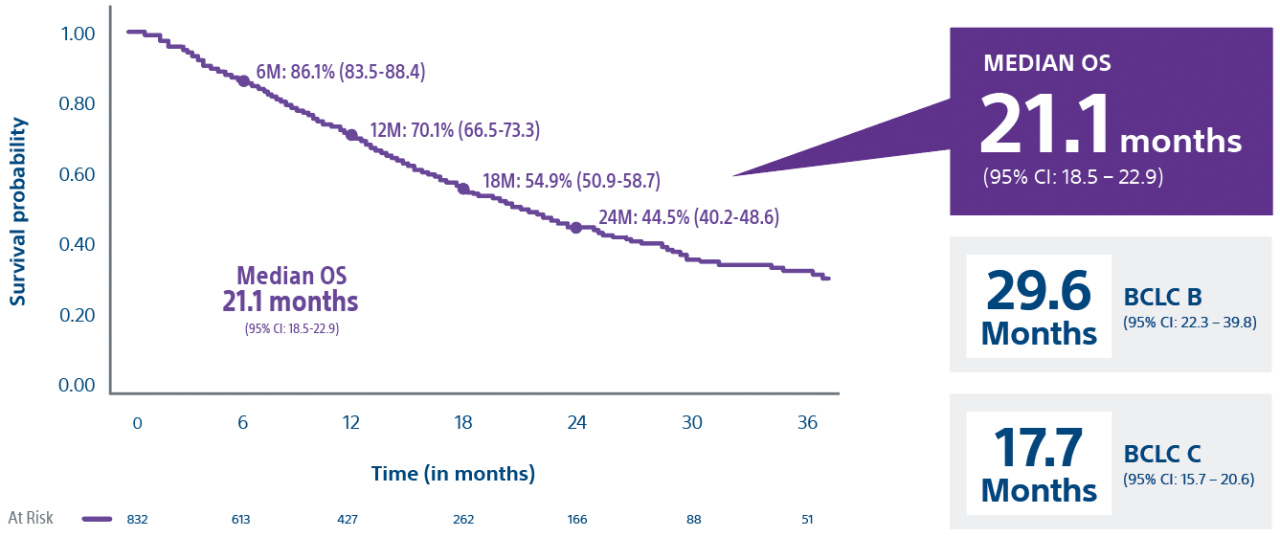
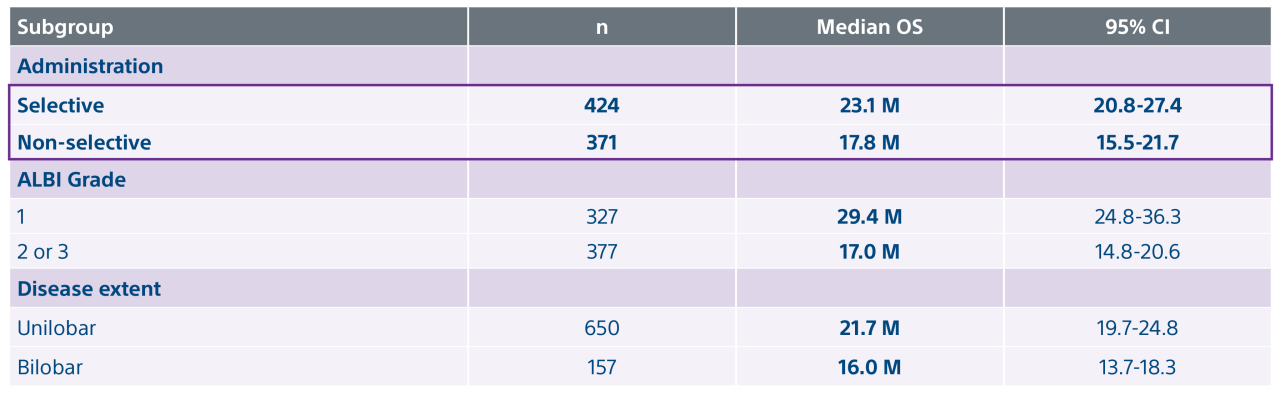
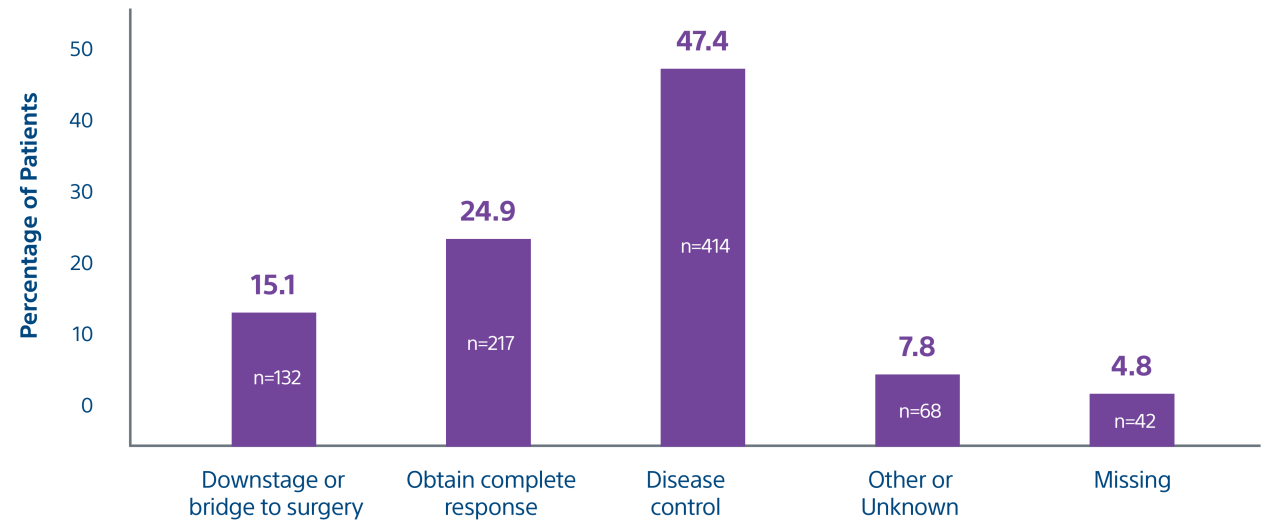
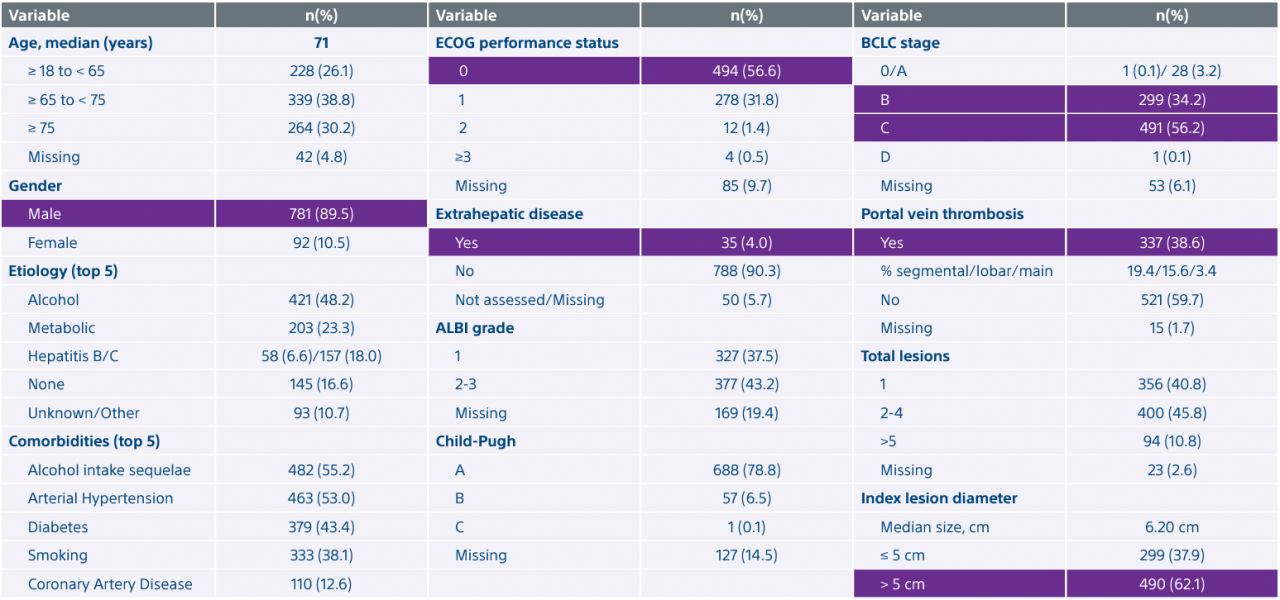
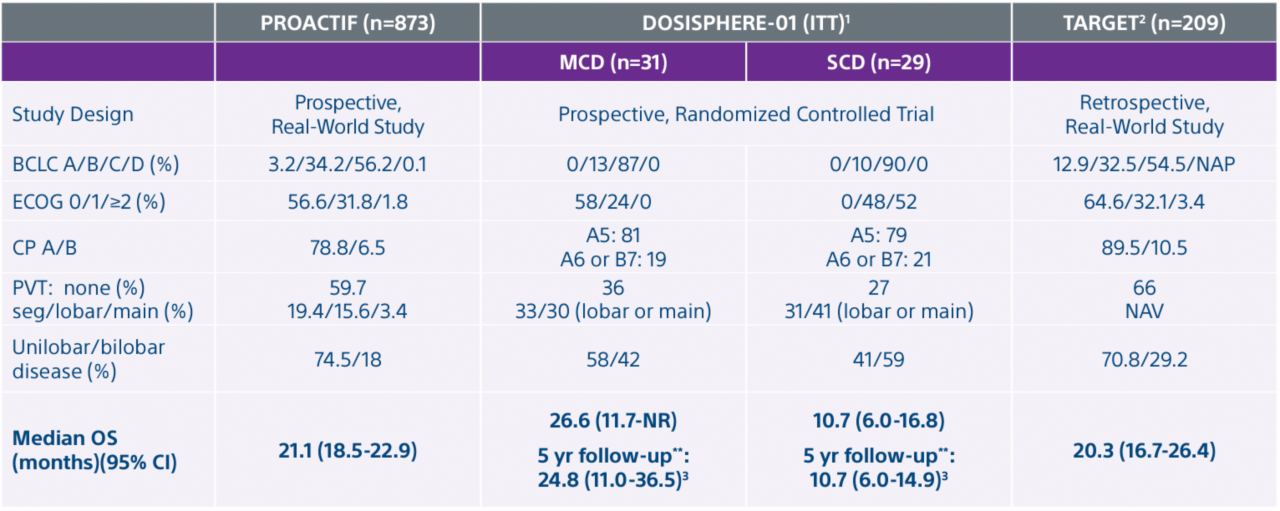
*Interim data presented at SIR 2024. Data collection as of 12/30/2023. Final results are expected in 2025
** Based on mITT population.
- Garin E, Tselikas L, Guiu B et al. Personalized versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021, 6: 17-29.
- Lam, M., Garin, E., Maccauro, M. et al. A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with Yttrium-90: the TARGET study. Eur J Nucl Med Mol Imaging (2022). https://doi.org/10.1007/s00259-022-05774-0.
- Garin E, Tselikas L, Guiu B, et al. Long-Term Overall Survival After Selective Internal Radiation Therapy for Locally Advanced Hepatocellular Carcinomas: Updated Analysis of DOSISPHERE-01 Trial. J Nucl Med. 2024;65(2):264-269. Published 2024 Feb 1. doi:10.2967/jnumed.123.266211
Garin E, Bailly C, Letang A et al. Abstract No. 257 • Featured Abstract The PROACTIF French Registry Study of Y-90 Glass Microspheres for the Treatment of Liver Malignancies: Interim Analysis of 670 Hepatocellular Carcinoma (HCC) Patients. J Vasc Interv Radiol. 2024 Mar 35(3):S113 doi: https://doi.org/10.1016/j.jvir.2023.12.295 (updated dataset for oral presentation at SIR March 2024)