Obsidio Embolic Limited Market Evaluation (LME) report
Background – Obsidio received 510(k) clearance in July 2022 and Boston Scientific acquired Obsidio (formerly GEM/Gel Embolic Material) from Obsidio, Inc. in August 2022. Boston Scientific chose to launch Obsidio Embolic in the US through a limited market evaluation (LME) to obtain early user experience prior to moving into a full commercial launch.
Methods – 27 sites in the US were chosen for the LME. Post completion of each case, the commercial representatives were tasked with completion of a case report survey.
Technical Success – Technical success for a Obsidio Embolic case was defined as successful embolization of the target vasculature. In the LME, Obsidio Embolic was able to achieve embolization target in all cases and had a success rate of 100% (131/131 cases).
OCCLUDE Registry Overview
Objective – Assess effectiveness and safety outcomes of patients who undergo embolization with Obsidio Conformable Embolic.
Planned number of site/subjects – Up to 125 patients, 23 sites in US.
Follow-up schedule – Pre-procedure, Index Procedure, discharge, 7-Day and 1-Month follow up visit (phone or in-office).
Interim Analysis (IA) – IA planned after 60 patients have completed follow up.
MD Anderson Publication Summary:
A Single-Center Experience with a Shear-Thinning Conformable Embolic
Recently published in JVIR, the retrospective study evaluated outcomes after embolization using Obsidio Embolic and demonstrated a treatment effectiveness rate of 100%.
Obsidio first-in-human clinical study: evaluation of Obsidio Embolic safety and performance
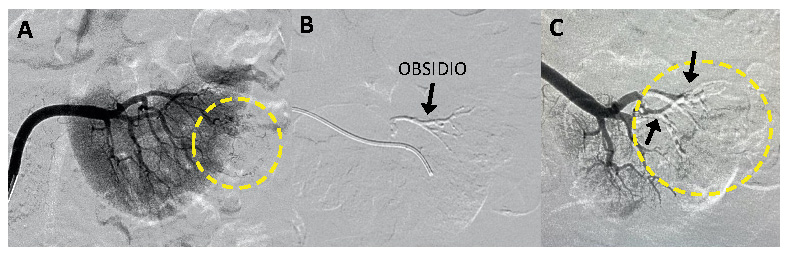
Overview – A single-center study* sought to evaluate the safety and performance of Obsidio Conformable Embolic (OCE) in patients requiring vascular embolization for hypervascular renal tumors.
Objectives – The study sought to demonstrate that Obsidio Embolic could be delivered safely, occludes the artery where deposited without migration, and does not lead to a change in standard blood tests.
Results – Obsidio Embolic achieved the absence of flow in the blood vessels of tumors, with embolization demonstrated to be durable without migration at Day 7.