Boston Scientific accounts are for healthcare professionals only.

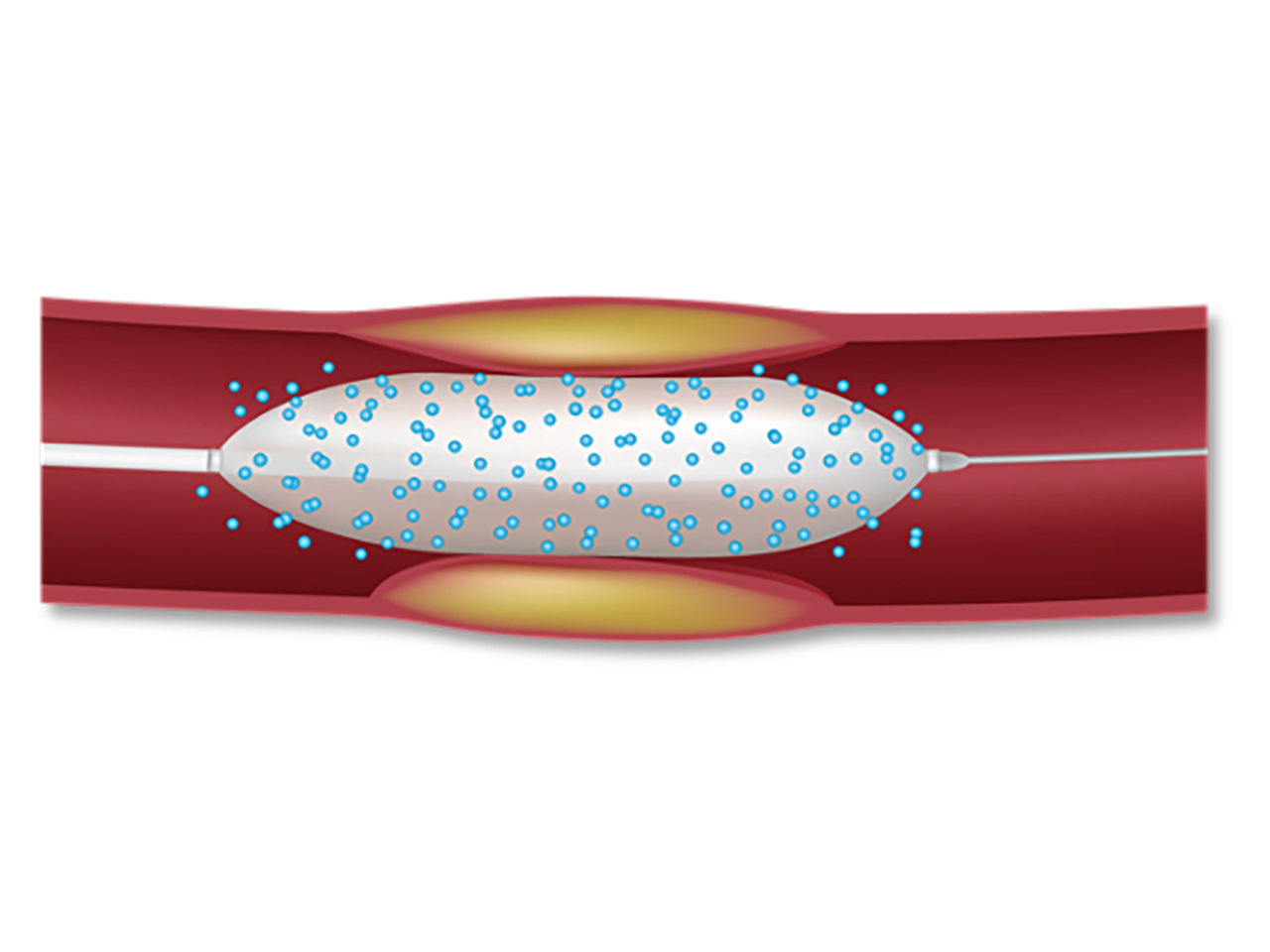
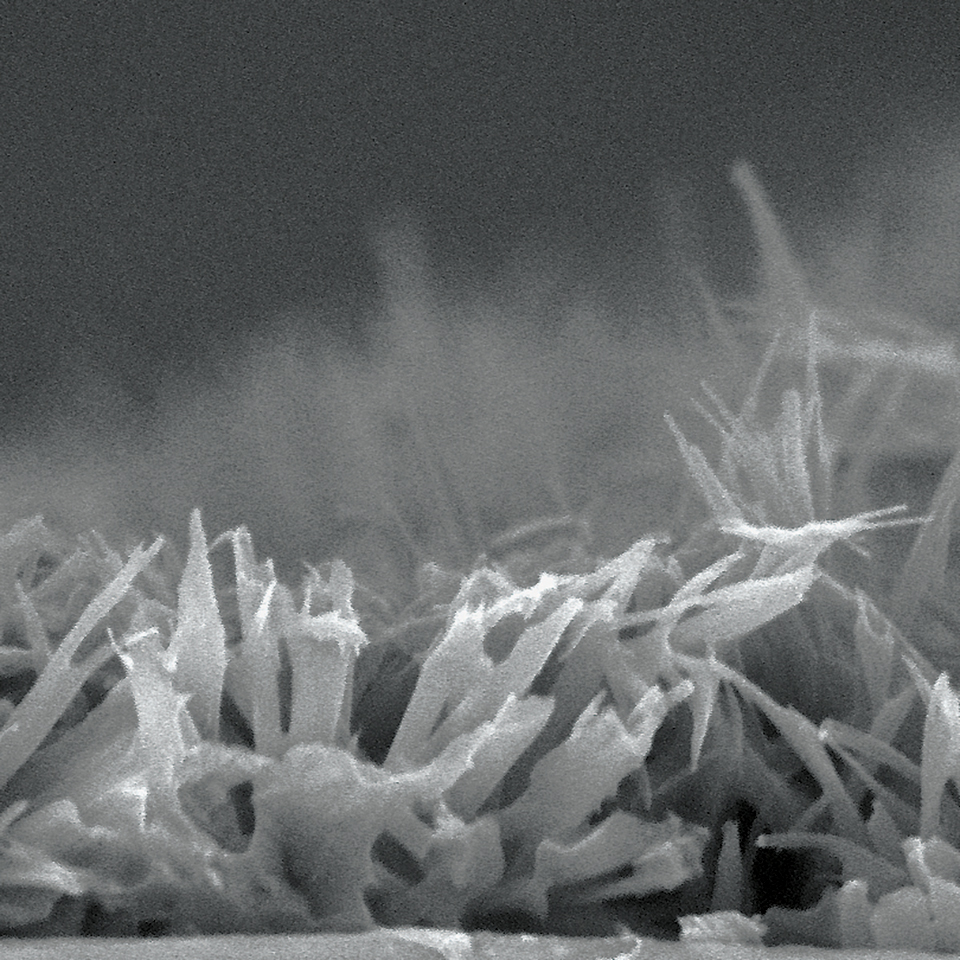
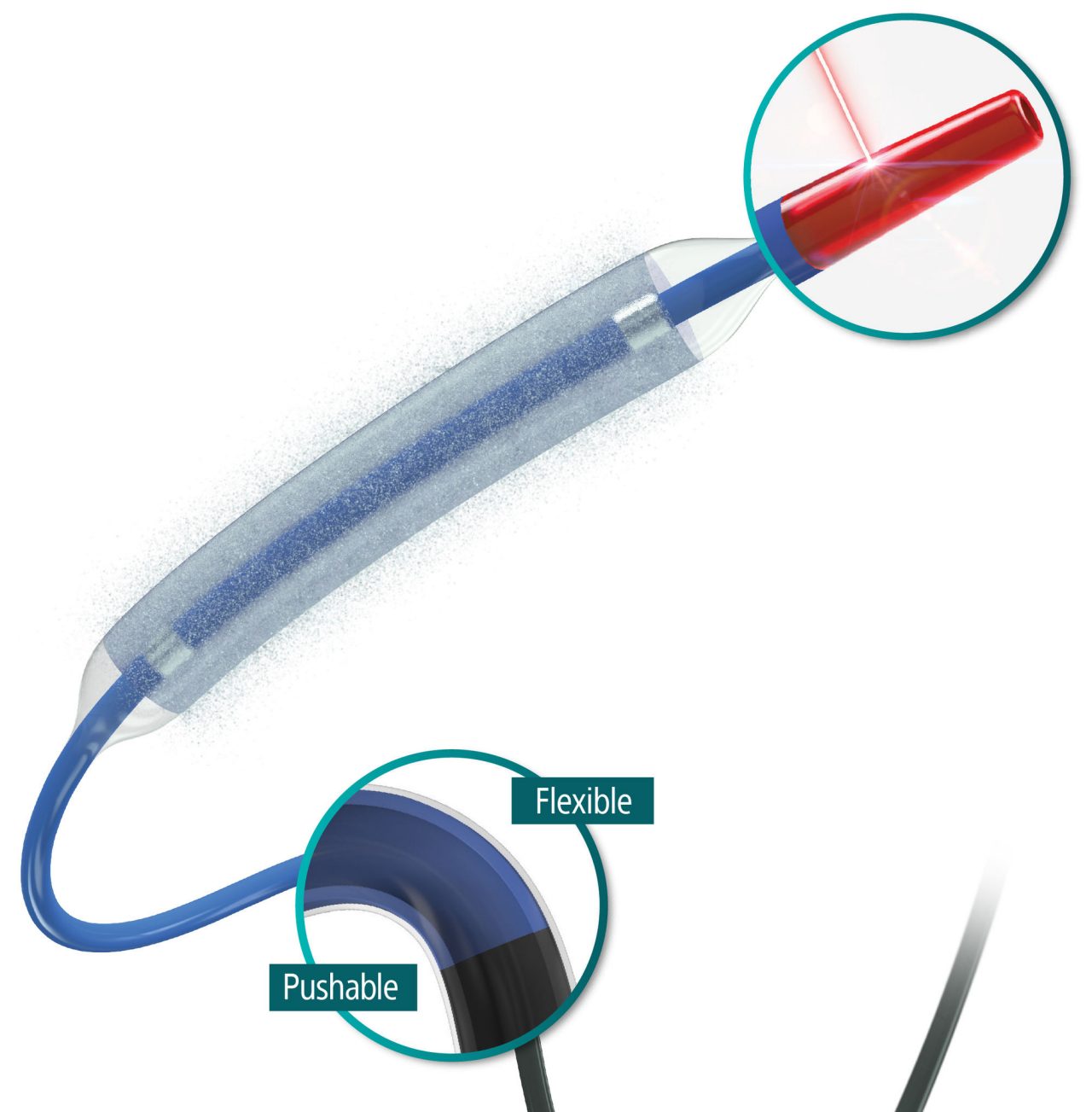
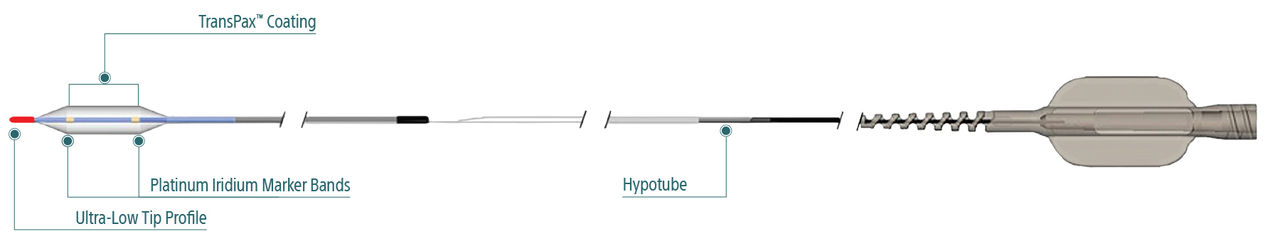
AGENT™ Drug-Coated Balloon
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Ordering information
- Training
- Reimbursement
- Resources
Clinical highlights
2024
AGENT IDE Trial
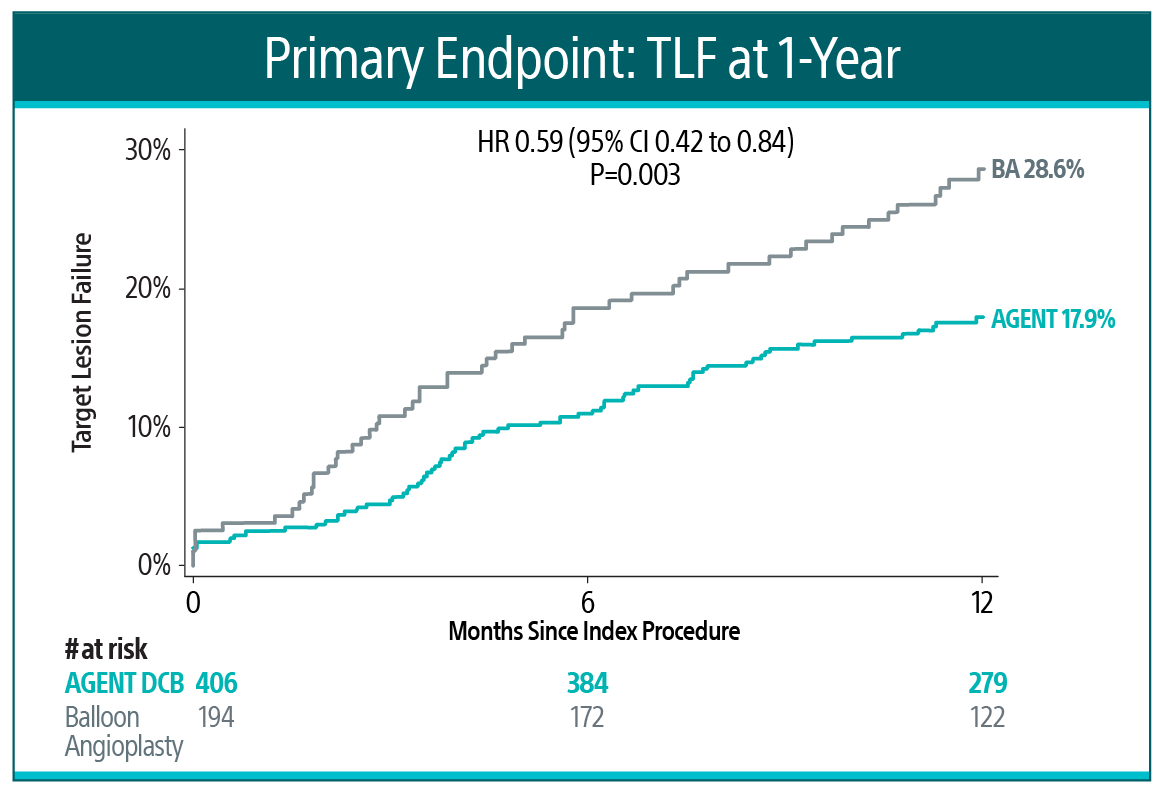
AGENT IDE is a prospective, multicenter, randomized controlled trial in the United States to evaluate the safety and effectiveness of the AGENT™ DCB compared to balloon angioplasty in patients with in-stent restenosis (ISR).15 At one year, AGENT DCB demonstrated statistically lower event rates15:
AGENT IDE Trial Primary Endpoint16
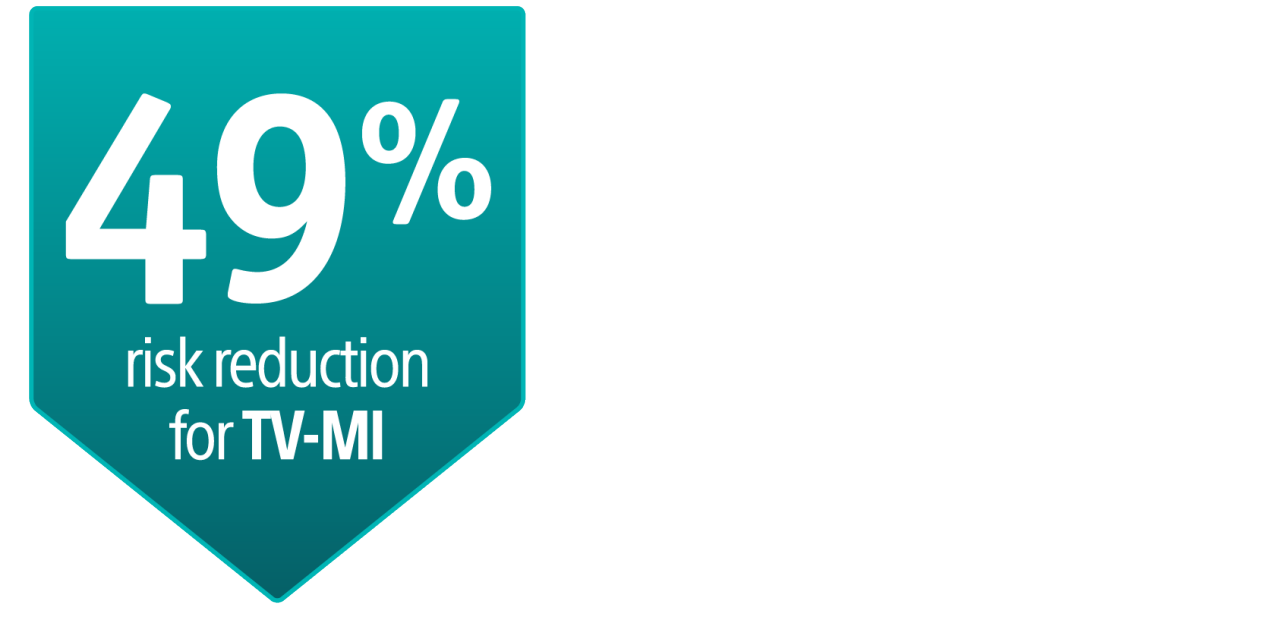
AGENT DCB showed statistically superior outcomes compared to balloon angioplasty for TLF at 1-year (17.9% versus 28.6% P= 0.003).
TLF relative risk reduction from using AGENT DCB was approximately 41%.

Featured clinical publications
The American College of Cardiology (ACC) Interventional Council
In February 2023, The American College of Cardiology (ACC) Interventional Council released the recommendation that all cardiac cath labs should have imaging capabilities. The review proposes PCI best practices and advocates for broader use of IVI technologies.
RENOVATE-COMPLEX-PCI study
The recent RENOVATE-COMPLEX-PCI study provides evidence in favor of using intravascular imaging-guided PCI to address complex coronary artery lesions. At the 3-year follow-up, intravascular imaging-guided PCI demonstrated a reduced risk of target vessel failure when compared to angiography-guided PCI.¹
Modern PCI clinical data
Discover more clinical data for intravacular ultrasound (IVUS), FFR, and vessel preparation.
1. Stolker JM, Cohen DJ, Kennedy KF, Pencina MJ, Lindsey JB, Mauri L, Cutlip DE, Kleiman NS. Evaluation of Drug-Eluting Stents and Ischemic Events (EVENT) Investigators. Repeat revascularization after contemporary percutaneous coronary intervention: An evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year
2. Moussa ID, Mohananey D, Saucedo J, et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76:1521–1531.
3. Kastrati A, Cassese S (2020). In-stent restenosis in the United States: Time to enrich its treatment armamentarium. In (Vol. 76, pp. 1532–1535): American College of Cardiology Foundation, Washington DC.
4. Tepe G, Brodmann M, Micari A, et al. 5-year outcomes of drug-coated balloons for peripheral artery in-stent restenosis, long lesions, and CTOs. JACC Cardiovasc Interv. 2023;16:1065–1078.
5. Kawamoto H, Ruparelia N, Latib A, et al. Drug-coated balloons versus second-generation drug-eluting stents for the management of recurrent multimetal-layered in-stent restenosis. JACC Cardiovasc Interv. 2015;8:1586–1594.
6. Yabushita H, Kawamoto H, Fujino Y, et al. Clinical outcomes of drug-eluting balloon for in-stent restenosis based on the number of metallic layers. Circ Cardiovasc Interv. 2018;11:e005935.
7. Market Data on file at BSC as of July 2023.
8. Nakamura M, Isawa T, Nakamura S, et al. Drug-coated balloon for the treatment of small vessel coronary artery disease – a randomized non-inferiority trial. Circ J. 2023;87:287–295.
9. Alfonso F, Coughlan JJ, Giacoppo D, Kastrati A, Byrne RA. Management of in-stent restenosis. EuroIntervention: Journal of EuroPCR in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2022;18:e103–e123.10. Market Data on file at BSC as of July 2023.
10. Surapaneni M, et al. International Scholarly Research Notices 2012.
11. Kim M, et al. International journal of nanomedicine (2011): 6:2997–3009. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process.
12. Teichgräber, U, et al. Head-to-head comparison of sirolimus- versus paclitaxel-coated balloon angioplasty in the femoropopliteal artery: study protocol for the randomized controlled SIRONA trial. Trials 22, 665 (2021)
13. Tzafriri A, et al. Journal of Controlled Release. 2019; 310:94–102. Taking paclitaxel coated balloons to a higher level: Predicting coating dissolution kinetics, tissue retention and dosing dynamics
14. Granada J. What’s next in drug-eluting SFA technology? Endovascular Today. 2017;16:9.
15. Yeh RW, Bachinsky W, Stoler R, et al. Rationale and design of a randomized study comparing the AGENT drug-coated balloon to plain old balloon angioplasty in patients with in-stent restenosis. Am Heart J. 2021;241:101–107.
16. AGENT IDE Clinical Trial data presented at CRT 2024 by Dr. Robert Yeh.
17. AGENT IDE Clinical Trial 2-year data presented at CRT 2025 by Dr. Jeffrey Moses.
18. Recurrent TLR calculated by the LWYY Method.
19 Medicare Hospital Outpatient Prospective Payment System (OPPS) Final Rule; CMS-1809-FC; p. 507; This document is scheduled to be published in the Federal Register on 11/27/2024 and available online at https://federalregister.gov/d/2024-25521.
20. https://www.cms.gov/files/zip/january-2025-alpha-numeric-hcpcs-file.zip
Please review the AGENT Brief Summary for full instructions on use.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. The material not intended for use in France.
All trademarks are the property of their respective owners.