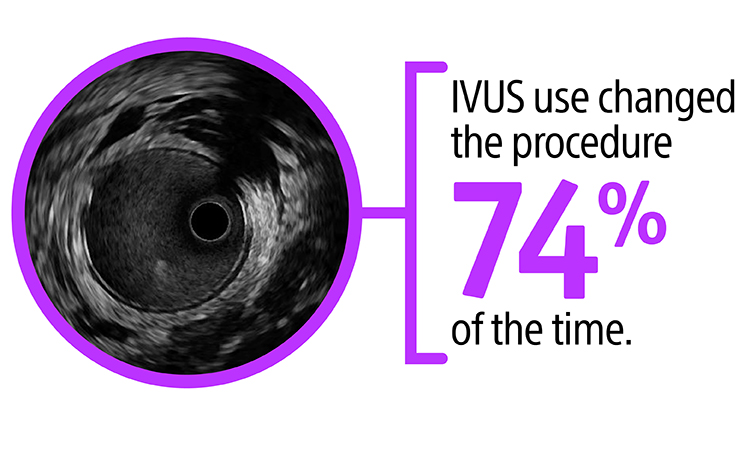
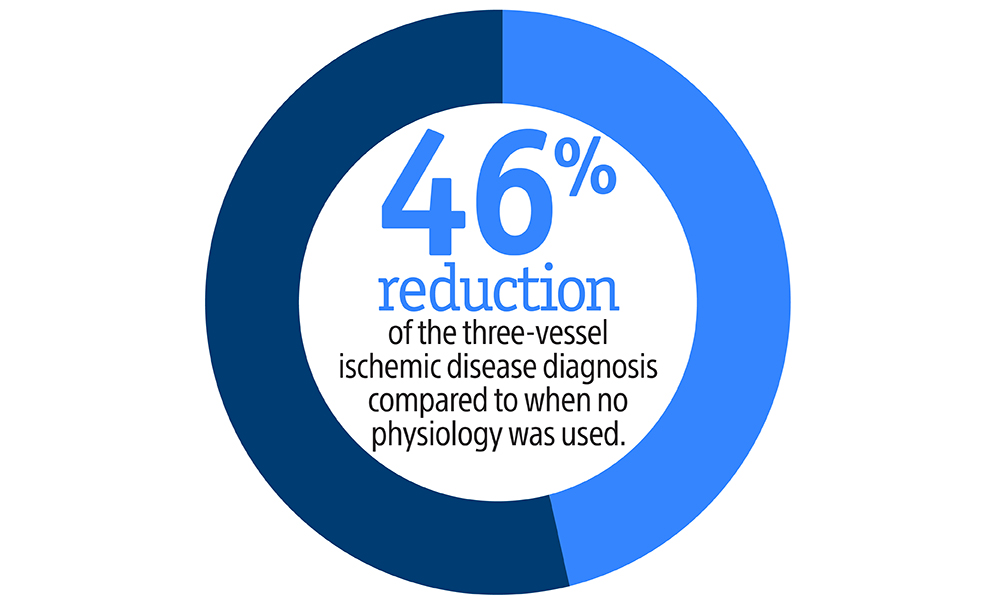
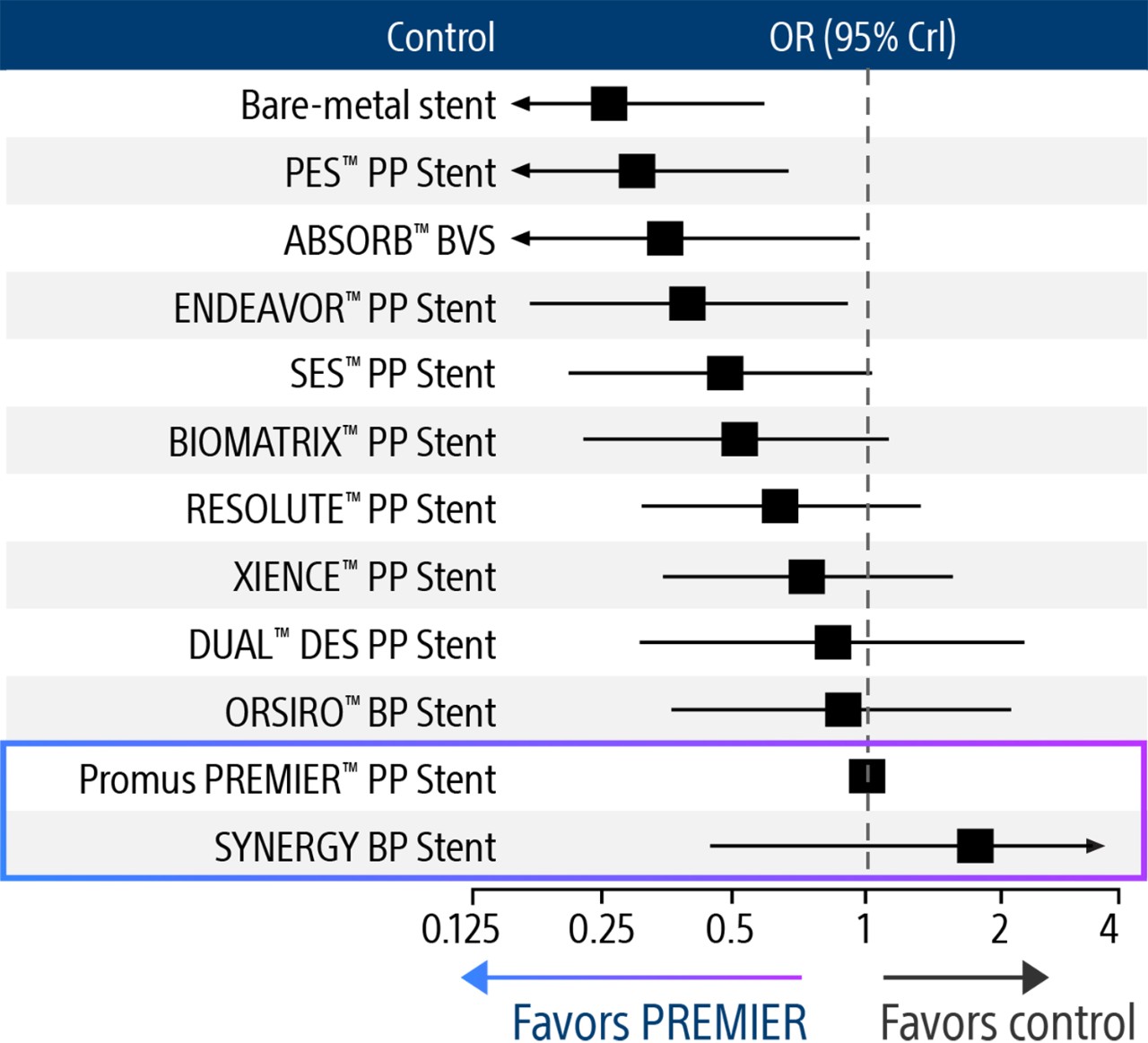
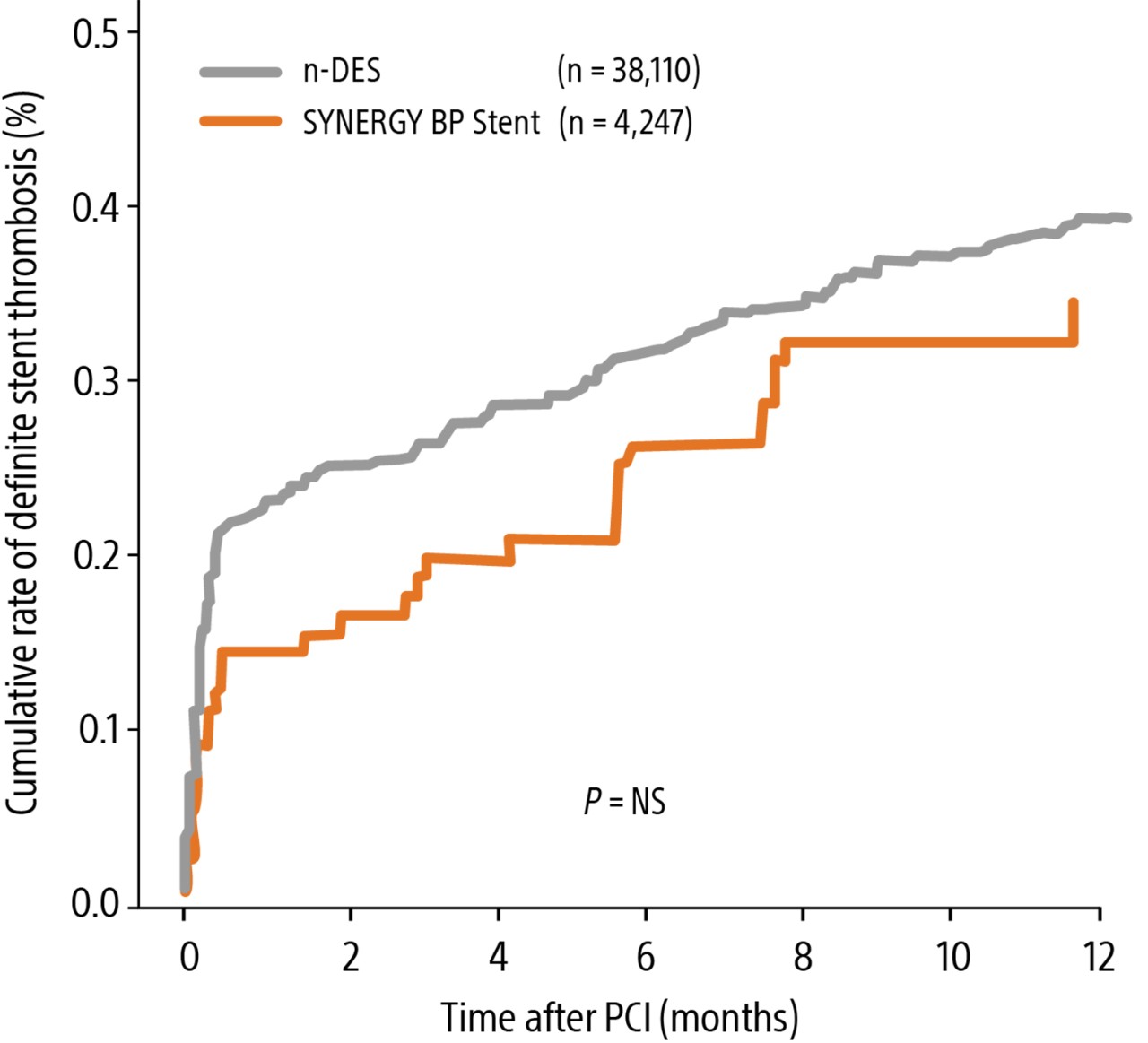
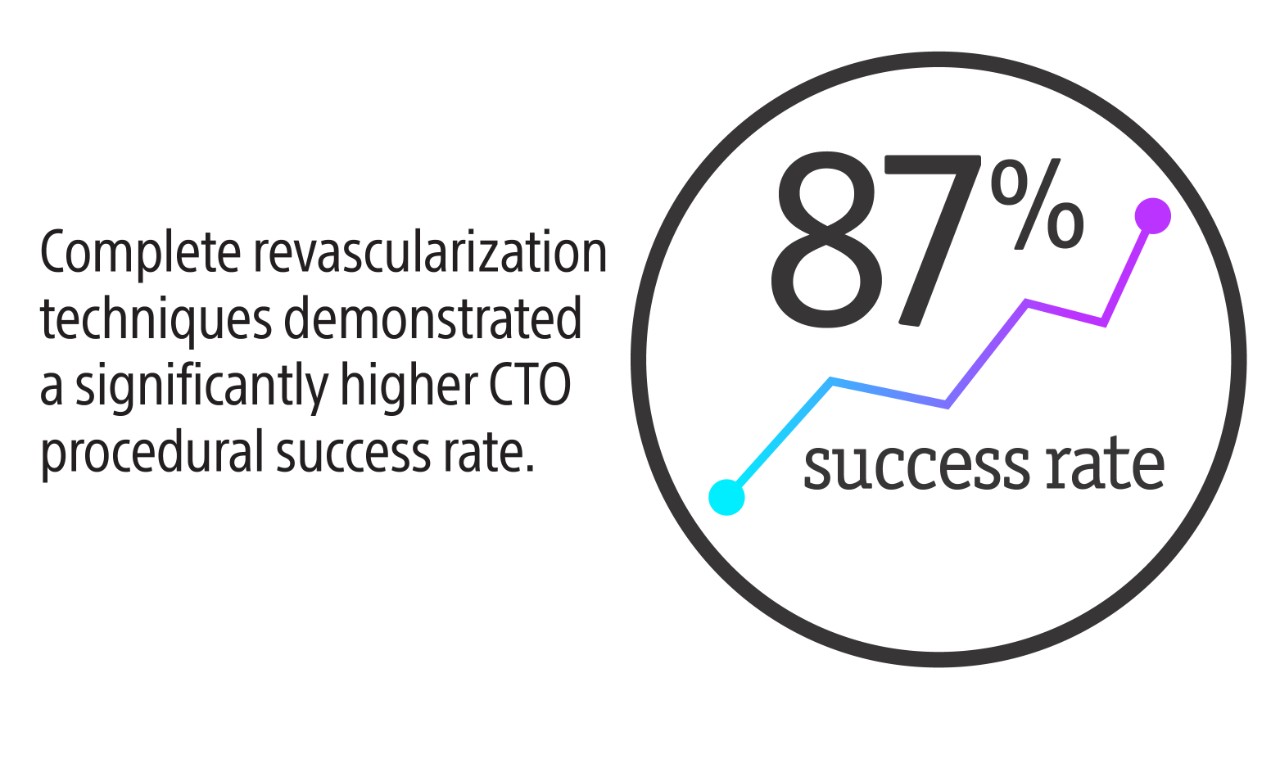
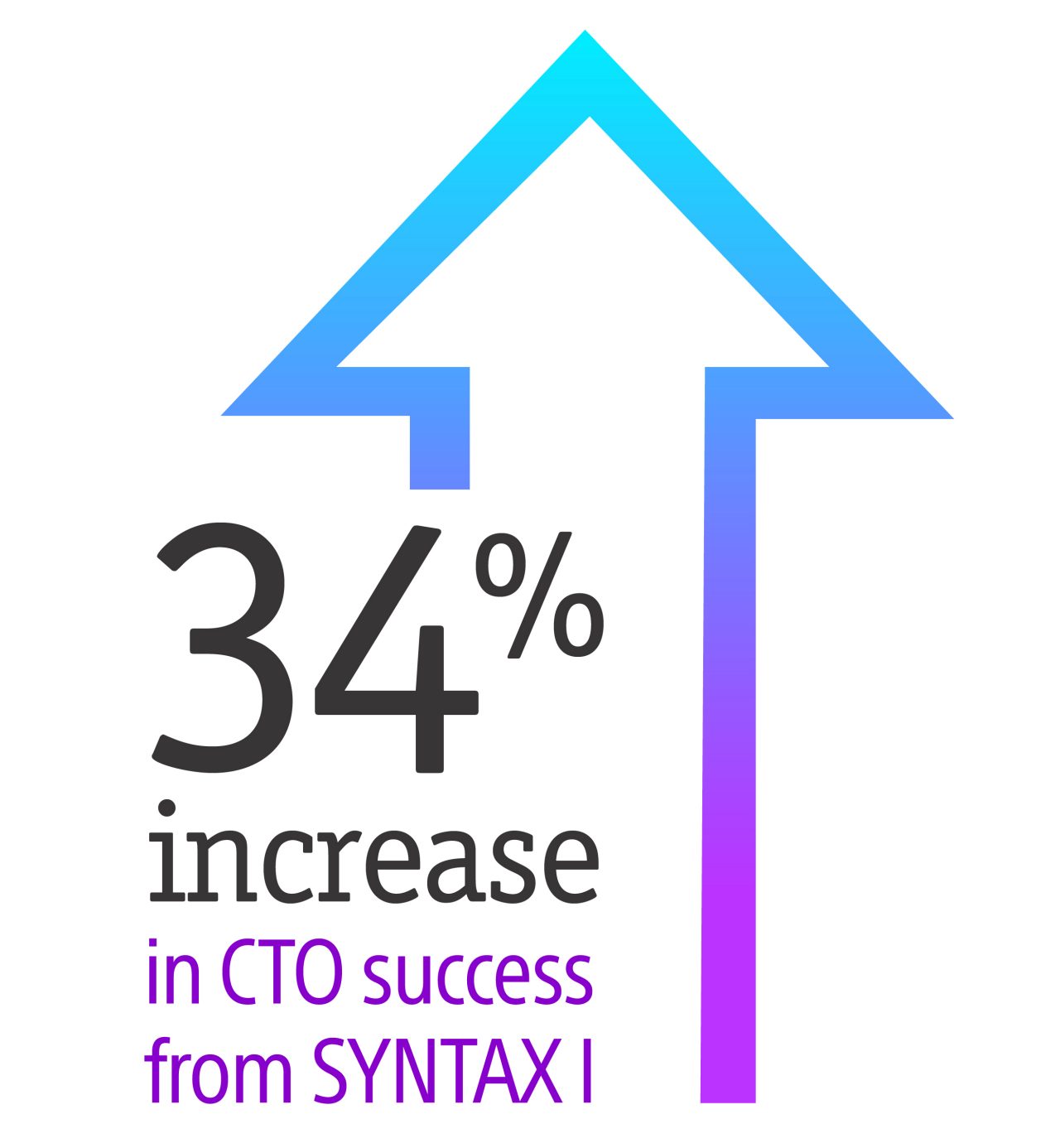
Nothing speaks louder than data
Whether you’re evaluating a new treatment, product, or technique, nothing speaks louder than clinical data. Every offering in our Percutaneous Coronary Interventions (PCI) portfolio represents a body of research spanning four decades. No matter what you need, our portfolio delivers the safety, efficacy, and performance you demand- backed by data you can trust.