Modern PCI means using all the newest data and tools at our disposal to provide the best possible treatment for coronary artery disease (CAD) patients. The latest SYNTAX II results, along with supporting clinical evidence, like the AGENT IDE Trial, show that integrating risk stratification, multidisciplinary collaboration, new tools & techniques, and innovative drug eluting technologies (DET) can lead to better outcomes -- even in the most complex cases.
An AGENT of change for ISR treatment
Data from the first coronary drug-coated balloon (DCB) study in U.S. met the 1-year primary endpoint and demonstrated low adverse event rates.
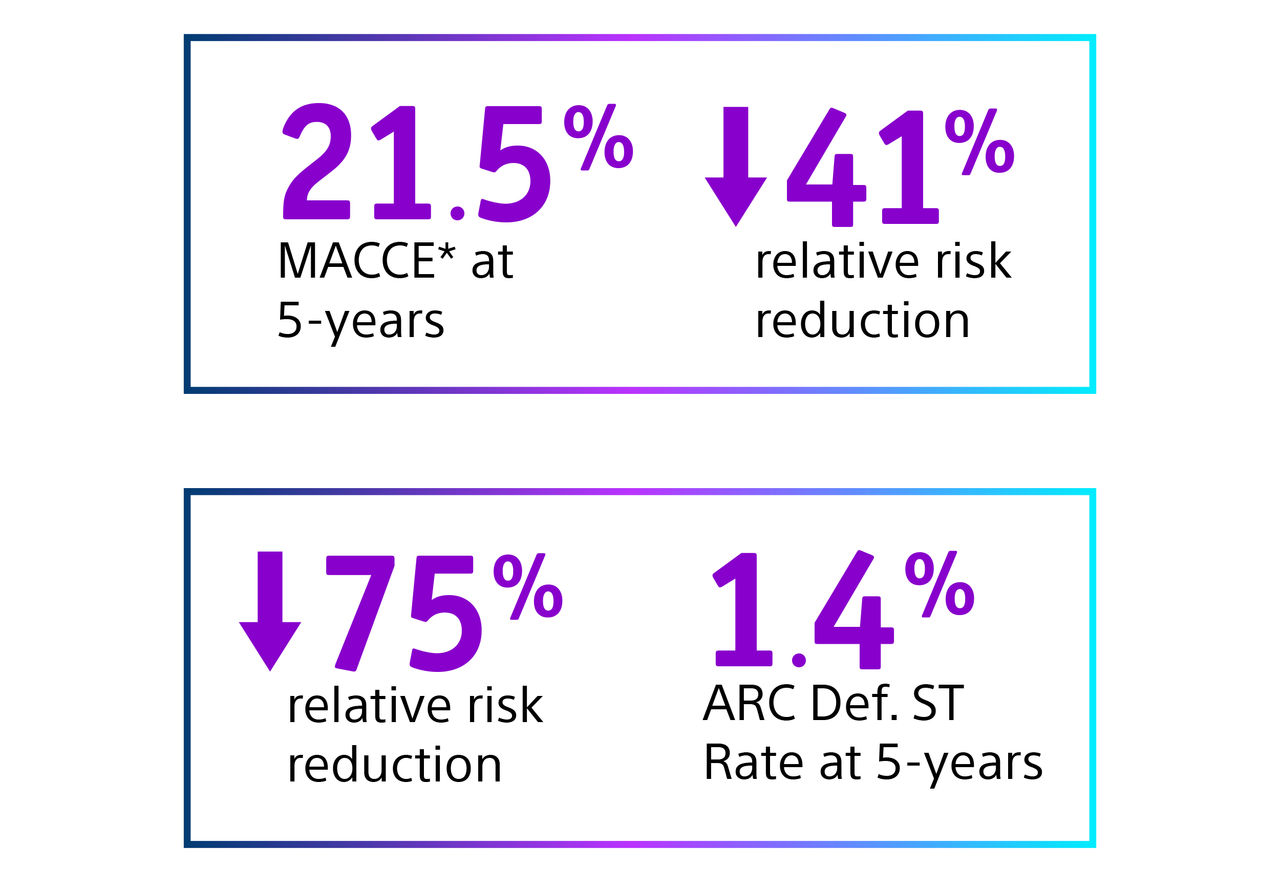
- Primary endpoint1 met! AGENT DCB showed statistically superior outcomes compared to balloon angioplasty for TLF at 1-year. (17.9% versus 28.6% P= 0.003).
- The TLF relative risk reduction from using AGENT DCB was approximately 41%.

The latest generation DES plays a role in elevating PCI outcomes
The CONSISTENT CTO2 study showed the use of up-to-date technologies and techniques in opening a CTO, including the use of an everolimus eluting stent with a bioabsorbable polymer, can result in significant quality of life improvements without significant impact on intravascular healing, even in very symptomatic patients with complex lesions.
Using the latest PCI technologies and techniques can drive substantial quality of life improvements.
Successful CTO PCI in 91% of patients.
Significant improvement in all SAQ components at 12 months.