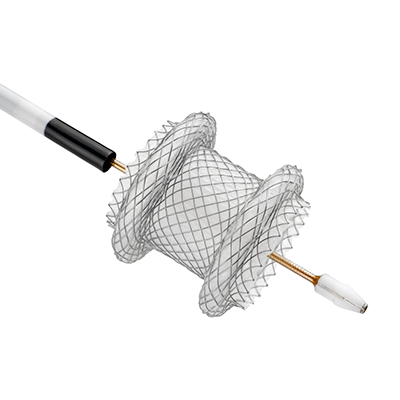
New indication for AXIOS for EUS-guided gallbladder drainage
AXIOS Stent technology is now FDA-cleared for transgastric or transduodenal endoscopic ultrasound (EUS)-guided gallbladder drainage (GBD) in high-risk patients with acute cholecystitis.* When surgery is not an option, EUS-guided GBD is a viable treatment alternative – offering non-surgical candidates effective care that can reduce pain and reinterventions while preserving quality of life.
How AXIOS Stent technology works
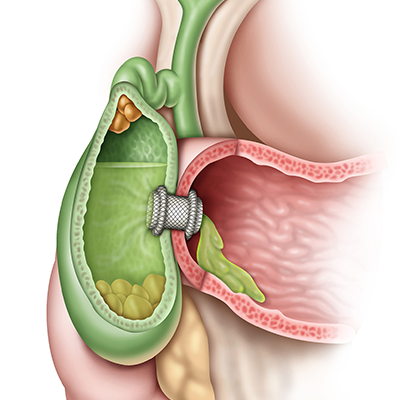
Manage acute cholecystitis
EUS-guided GBD can provide benefits for patients, clinical teams and hospitals.