Boston Scientific accounts are for healthcare professionals only.


SpaceOAR Vue™ System Perirectal Spacer for Prostate Radiation Therapy
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Ordering information
- Training
- Resources
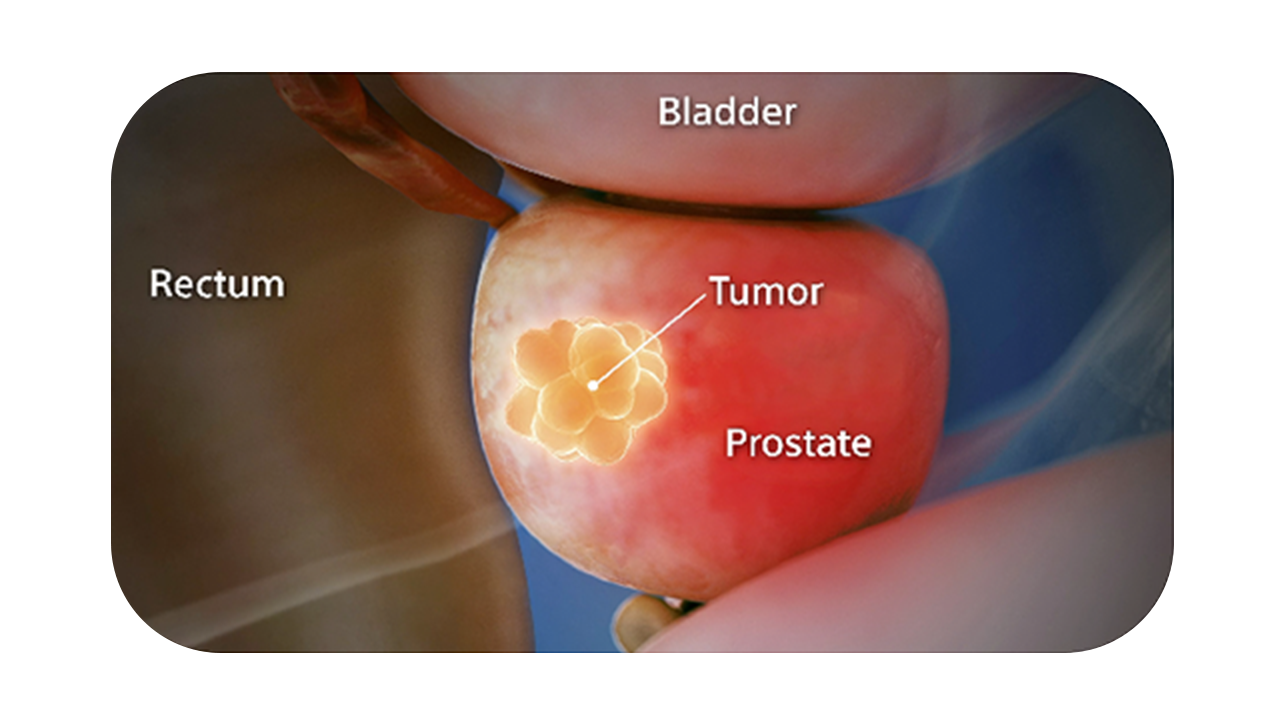
You see it when you CT it.
SpaceOAR Vue Hydrogel is the only FDA-cleared radiopaque PEG-based perirectal hydrogel spacer designed for CT visibility to help optimize radiotherapy treatment planning for prostate cancer.1
How it works
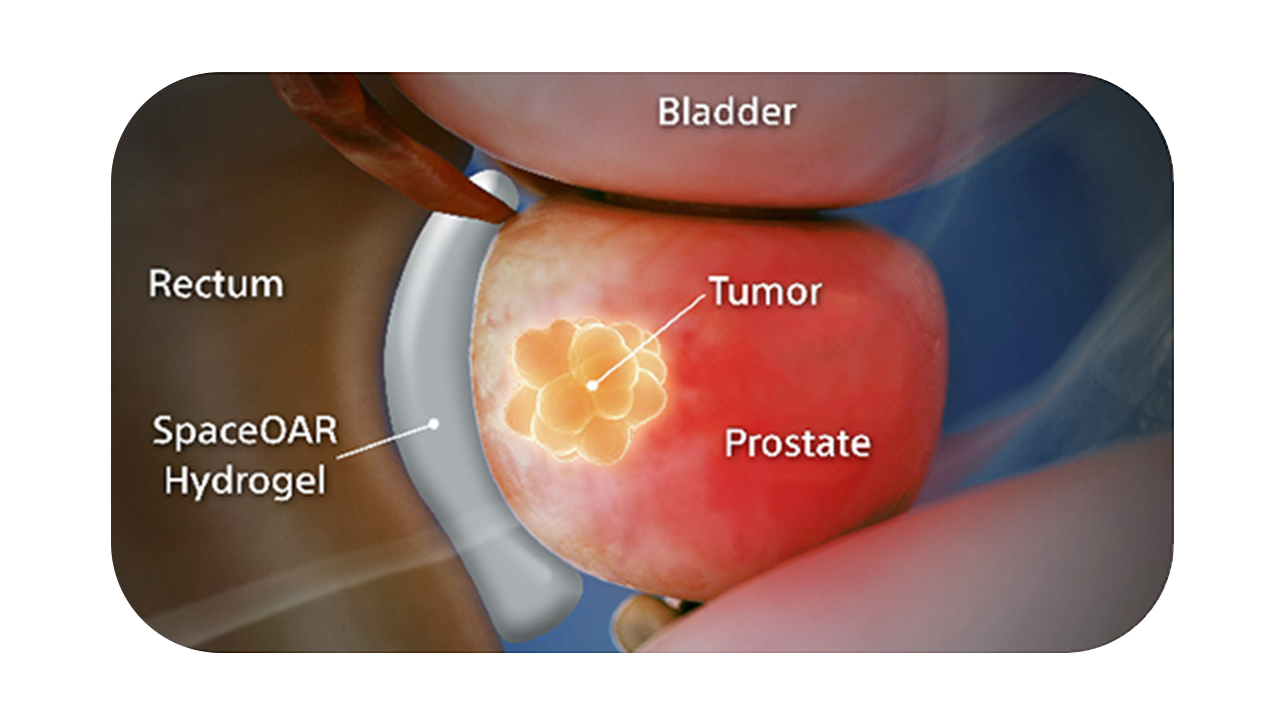
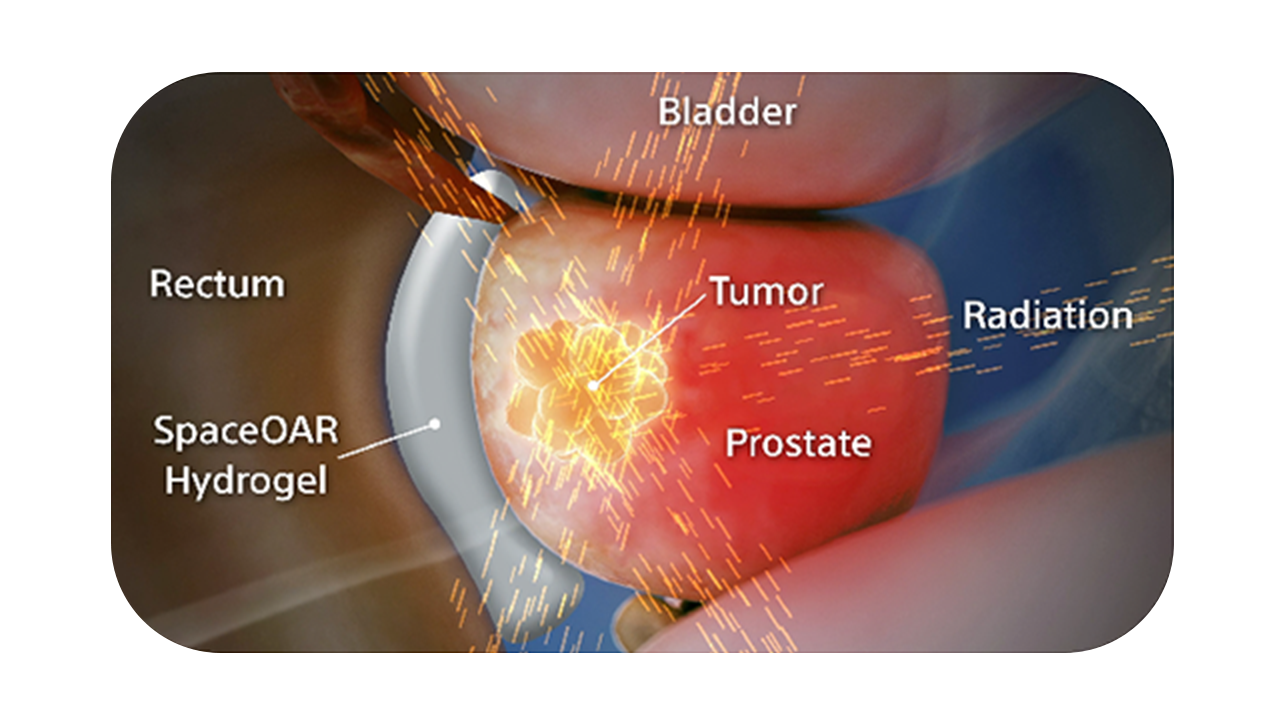
SpaceOAR Vue Hydrogel, the next-generation hydrogel perirectal spacer is designed to offer enhanced visibility on CT. Due to the close proximity between the rectum and the prostate, radiation therapy can unintentionally cause rectal toxicity. SpaceOAR Vue Hydrogel is designed to help reduce the radiation delivered to the rectum during treatment.1
Why choose SpaceOAR Vue Hydrogel
The radiopacity is designed to improve the contouring accuracy during treatment plan creation, when compared to SpaceOAR Hydrogel.1
Integrating SpaceOAR Vue Hydrogel into your practice
Boston Scientific supports a thorough in-service training program for office staff, clinical staff, and physicians in the practice. A Boston Scientific representative brings the training to you, and it can be completed in approximately 1 hour for each group.
Reference
- Data on file with Boston Scientific.
SpaceOAR Vue Hydrogel is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR Vue Hydrogel to reduce the radiation dose delivered to the anterior rectum.
SpaceOAR Vue Hydrogel contains polyethylene glycol (PEG) and iodine.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, and potential adverse events.
As with any medical treatment, there are some risks involved with the use of SpaceOAR Vue Hydrogel. Potential complications associated with SpaceOAR Vue Hydrogel include, but are not limited to: pain associated with SpaceOAR Vue Hydrogel injection, pain or discomfort associated with SpaceOAR Vue Hydrogel, local inflammatory reactions, infection (including abscess), urinary retention, urgency, constipation (acute, chronic, or secondary to outlet perforation), rectal tenesmus/muscle spasm, mucosal damage, ulcers, fistula, perforation (including prostate, bladder, urethra, rectum), necrosis, allergic reaction (localized or more severe reaction, such as anaphylaxis), embolism (venous or arterial embolism is possible and may present outside of the pelvis, potentially impacting vital organs or extremities), syncope, and bleeding. The occurrence of one or more of these complications may require treatment or surgical intervention. URO-989810-AB
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.