Boston Scientific accounts are for healthcare professionals only.
Boston Scientific accounts are for healthcare professionals only.
Create an account to access online training and education on EDUCARE, manage your customer profile, and connect with customer support and service teams.
My Boston Scientific account
Access your online applications and manage your customer profile.
Quick Links
Call customer care

Whether you're looking to get started with SpaceOAR Hydrogel or have general questions about the product, procedure, or clinical data, our team is here to help.
When you choose SpaceOAR Hydrogel for your prostate cancer patients, you’re not only getting a device with proven clinical outcomes — you’re choosing a world-class customer experience.

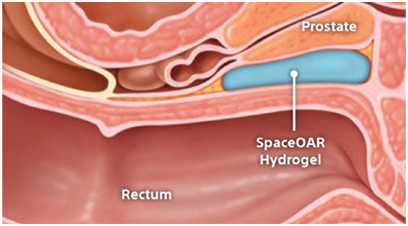
For prostate cancer patients undergoing radiation therapy, maintaining quality of life may be just as important as treating the cancer.
*Of men who had erections sufficient for intercourse at baseline.
†Number of patients is based on units shipped and a BSC proprietary algorithm.
‡Data based on sales for SpaceOAR™ Hydrogel or SpaceOAR Vue™ Hydrogel to institutions in 2022 and 2023
**CPT copyright © 2023 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association.
References
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
SpaceOAR and SpaceOAR Vue Hydrogels are intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR and SpaceOAR Vue Hydrogels to reduce the radiation dose delivered to the anterior rectum.
SpaceOAR and SpaceOAR Vue Hydrogels contain polyethylene glycol (PEG). SpaceOAR Vue Hydrogel contains iodine.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
As with any medical treatment, there are some risks involved with the use of SpaceOAR and SpaceOAR Vue Hydrogels. Potential complications associated with SpaceOAR and SpaceOAR Vue Hydrogels include, but are not limited to: pain associated with SpaceOAR and SpaceOAR Vue Hydrogels injection, pain or discomfort associated with SpaceOAR and SpaceOAR Vue Hydrogels, local inflammatory reactions, infection (including abscess), urinary retention, urgency, constipation (acute, chronic, or secondary to outlet perforation), rectal tenesmus/muscle spasm, mucosal damage, ulcers, fistula, perforation (including prostate, bladder, urethra, rectum), necrosis, allergic reaction (localized or more severe reaction, such as anaphylaxis), embolism (venous or arterial embolism is possible and may present outside of the pelvis, potentially impacting vital organs or extremities), syncope and bleeding. The occurrence of one or more of these complications may require treatment or surgical intervention. URO-989811-AB.
Health economic and reimbursement information provided by Boston Scientific Corporation is gathered from third-party sources and is subject to change without notice as a result of complex and frequently changing laws, regulations, rules, and policies.
This information is presented for illustrative purposes only and does not constitute reimbursement or legal advice.
Boston Scientific encourages providers to submit accurate and appropriate claims for services. It is always the provider’s responsibility to determine medical necessity, the proper site for delivery of any services, and to submit appropriate codes, charges, and modifiers for services rendered. It is also always the provider’s responsibility to understand and comply with Medicare national coverage determinations (NCD), Medicare local coverage determinations (LCD), and any other coverage requirements established by relevant payers which can be updated frequently. Boston Scientific recommends that you consult with your payers, reimbursement specialists, and/or legal counsel regarding coding, coverage, and reimbursement matters.
Boston Scientific does not promote the use of its products outside their FDA-approved label.
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements.
CPT Copyright © 2023 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. Applicable FARS/DFARS Restrictions Apply to Government Use. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein.” All trademarks are the property of their respective owners.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.