ANGIOJET™ Ultra
Coronary Thrombectomy System
The ANGIOJET Ultra Thrombectomy System is a mechanical thrombectomy device for PCI patients with large thrombus burden and the ONLY FDA-approved mechanical thrombectomy device for removal of thrombus in coronary arteries. The system is designed to mechanically restore blood flow of patients with thrombosed arteries.
Key Resources
Explore
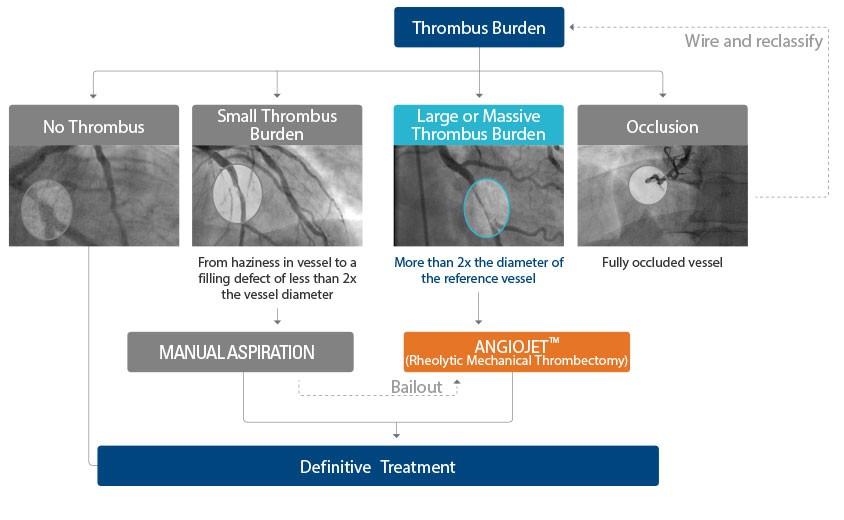
Assessing the level of thrombus burden and determining the treatment pathway¹
Product Details
Powerful mechanical thrombectomy performance
- Proven: The leading choice for large thrombus evacuation, utilized in more than 600,000 cases worldwide
- Versatile: Able to meet your preferences and a broad range of clinical scenarios with a wide range of catheters
Simple set-up
- Easy 3-step set-up: Preparation, loading and priming are straightforward, even for new users
- Compact design: The highly portable design enhances ease of integration in the hospital setting
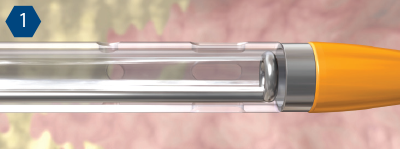
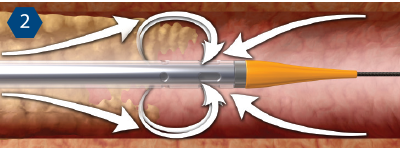
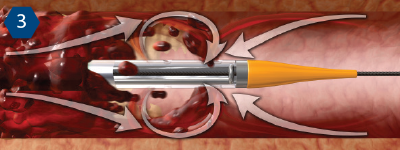
Mechanism of Action
Your single source for a full offering of thrombectomy solutions
Only Boston Scientific supplies thrombectomy solutions for every level of thrombus burden, with proven performance and minimal complications.
Case Study
SPIROFLEX™ ANGIOJET thrombectomy of a native coronary artery in a patient presenting with a ST elevation myocardial infarction (STEMI) due to stent thrombosis.
Patient History
- 52 year old male
- Former smoker, diabetes, hypertension, hyperlipidemia
- History of coronary artery disease
- Prior stent to the LAD and D1
- Prior stent to the circumflex
- Prior stent to the RCA
- Inferior MI secondary to stent thrombosis in January 2014 was treated with a second layer of stents in the proximal, mid, and distal RCA
- Presented with a second stent thrombosis of the RCA in January 2015 which was treated with PTCA and stenting of the mid RCA (3rd layer)
- Patient presented with chest pressure and inferior ST elevation approximately 3 months later
- Patient was referred for coronary angiography
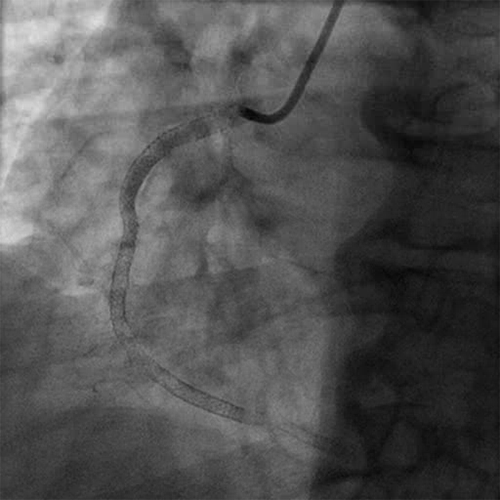
Diagnostic Angiogram
- Initial angiogram showed TIMI 0 flow
- Initial angiogram showed thrombus grade of 5
- The left main and left anterior descending arteries had no significant lesions. The stents in the LAD and D1 were patent
- The circumflex had no significant lesions. The stent in the first OM had a 50% lesion and the stents in the mid circumflex were patent
- The RCA was occluded in the mid vessel
Procedure
- The patient was anticoagulated with heparin and Integrilin™
- Vascular access was gained utilizing a 6 F JR4 guiding catheter and a 190 cm wire
- Post wire TIMI flow was 0; post wire thrombus grade was 5
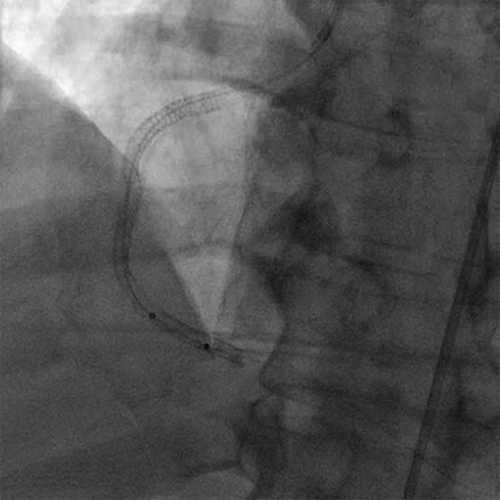
- The RCA was treated with ANGIOJET thrombectomy utilizing the SPIROFLEX catheter
- There was residual thrombus in the mid and distal stents
- Initially the mid and distal RCA into the posterior lateral artery were treated with multiple SPIROFLEX passes for a total of 27.5 seconds
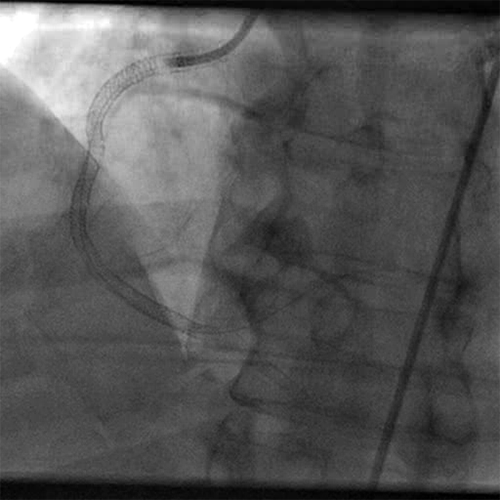
- A second series of runs was performed utilizing SPIROFLEX ANGIOJET for a total of 35 seconds
- Significant angiographic improvement was observed post mechanical thrombectomy
- Post ANGIOJET thrombus grade was 3
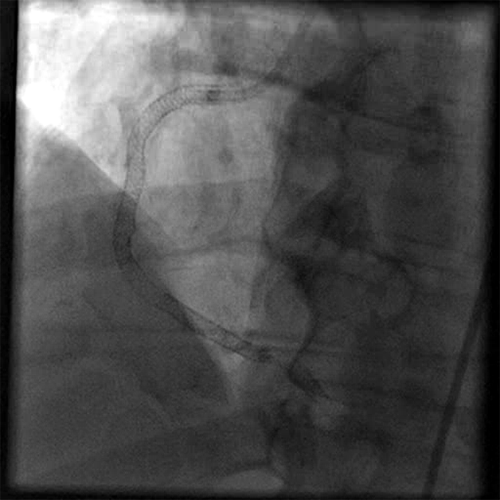
Definitive Treatment
Following PTCA with stenting, final TIMI flow was 3; final thrombus grade was 0.
Physician Commentary
As in this case, patients presenting with recurrent thrombosis over a long stented segment often have a large thrombus burden.
The ANGIOJET Thrombectomy System is ideal to treat patients with a considerable amount of large thrombus and in this patient the ANGIOJET successfully removed a significant thrombus from the mid and distal RCA making definitive treatment easier and reestablishing TIMI 3 flow.
This case demonstrates the utility of mechanical thrombectomy with the ANGIOJET SYSTEM in a patient presenting with a STEMI secondary to stent thrombosis and a substantial thrombus burden in the culprit vessel.
Ordering Information
| Product Name | Catalog Number | UPN |
|---|---|---|
| Spiroflex™ | 106553 | 106553-001 |
| Spiroflex™ VG | 106608 | 106608-001 |
Reimbursement
The C-Code used for the ANGIOJET Thrombectomy System is C1757. C-Codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific Corporation is not responsible for correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.