WallFlex™
Esophageal Stents
Our WallFlex Esophageal Stents deliver luminal patency in patients with esophageal strictures caused by intrinsic and/or extrinsic malignant tumors, through combination of flexibility and control for optimized patient care. View new clinical data below.
Clinical Information
New study results found that placement of a WallFlex Fully Covered Esophageal Stent resulted in safe and effective palliation of malignant dysphagia
Dr. Allesandro Repici et al. authored the largest study on the WallFlex Fully Covered Esophageal Stent, which was published in the December 2014 issue of Digestive and Liver Disease Journal. The goal of the study was to assess the clinical (recurrent dysphagia) and technical success using the WallFlex Fully Covered Esophageal Stent in patients with dysphagia due to primary inoperable esophageal cancer.
The study found that placement of a WallFlex Fully Covered Esophageal Stent resulted in safe and effective palliation of malignant dysphagia. The median dysphagia scores improved from “3” (ability to swallow liquids only) to “1” (ability to eat some solid food) at both fourteen days and four weeks after stent placement. In addition, twenty-four of the eighty-two patients (29.2%) went on to receive chemo- and/or radiotherapy after stent placement.
The article, Management of Inoperable Malignant Oesophageal Strictures with Fully Covered WallFlex Stent: A multicentre Prospective Study is available at https://www.ncbi.nlm.nih.gov/pubmed/25262010.
Product Details
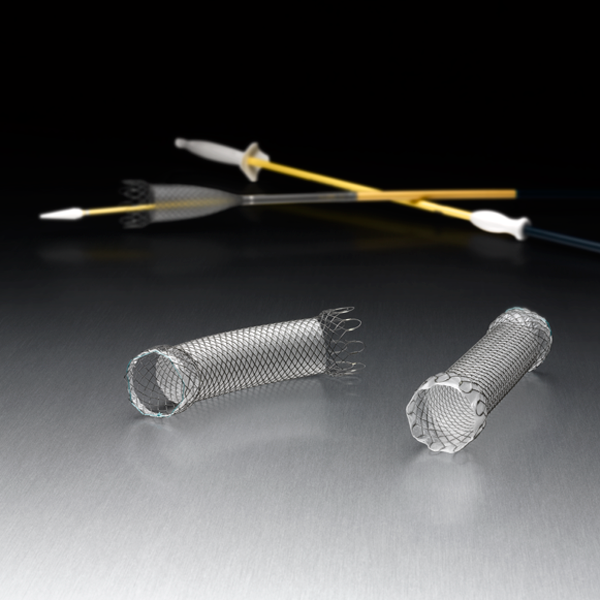
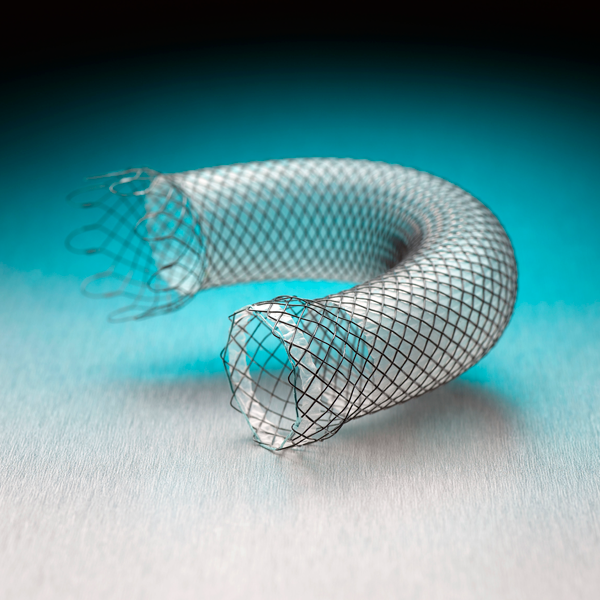
Building on the best of Boston Scientific’s industry-leading stents, the WallFlex Stent seeks to deliver luminal patency through a combination of flexibility and control to support your goal of optimized patient care.
The WallFlex Esophageal Stent is available in fully and partially covered options and a variety of lengths and widths.
WallFlex Esophageal Stent Details
Migration Resistance
The Progressive Step Flared Ends may assist in anchoring the stent within the esophageal lumen.
Stricture Resolution
The multiple wire braided construction is engineered to allow the WallFlex Esophageal Stent to adjust to forces from the esophageal anatomy such as strictures and peristalsis. The design allows for gradual stent expansion, which is typically complete after 24-72 hrs.
Tissue In-Growth Prevention
The Permalume™ Silicone Covering extends the entire length of the WallFlex Esophageal Stent in the fully covered version and is designed to prevent tumor ingrowth as well as stent concurrent esophageal fistulas and help reduce food impaction.
Adjustability
The Teflon™ Coated Polyester Removal Suture facilitates removal during the initial stent placement procedure.
Fluoroscopic Visualization
The Nitinol construction allows for clear visualization during fluoroscopy, ensuring accurate stent placement.
Delivery System Details
Pre-dilation Avoidance
The 18.5 French (6.2mm), low profile delivery system is designed to traverse tight strictures.*
Endoscopic Placement
The Endoscopic Transition Zone is designed to aid in stent placement accuracy when deployed using endoscopic visualization.
Stent Placement Accuracy
The coaxial delivery system is designed to result in 1:1 stent deployment.
The fully covered stent may be reconstrained up to 75% of deployment and 2 times during the initial
stent placement procedure.**
* Pre-dilation may not be required, depending on stricture lumen diameter.
** A stent can not be reconstrained after the reconstrainment limit has been exceeded.
Ordering Information
WallFlex™ Esophageal Stents
Magnetic Resonance (MR) Conditional
Non-clinical testing has demonstrated that the WallFlex Esophageal Stent System is MR Conditional. It can be scanned safely under the conditions outlined in the Directions For Use.

| Order Number | Stent O.D. (mm) | Proximal / Distal Flares O.D. (mm) | Stent Length (cm) | Catheter Diameter (Fr / mm) | Working Length (cm) | Packaging |
|---|---|---|---|---|---|---|
| M00516700 | 18 | 25 / 23 | 10.3 | 18.5 / 6.17 | 78 | Each |
| M00516710 | 18 | 25 / 23 | 12.3 | 18.5 / 6.17 | 78 | Each |
| M00516720 | 18 | 25 / 23 | 15.3 | 18.5 / 6.17 | 78 | Each |
| M00516730 | 23 | 28 / 28 | 10.5 | 18.5 / 6.17 | 78 | Each |
| M00516740 | 23 | 28 / 28 | 12.5 | 18.5 / 6.17 | 78 | Each |
| M00516750 | 23 | 28 / 28 | 15.5 | 18.5 / 6.17 | 78 | Each |
*Recommended Guidewire .038" Jagwire™ Guidewire, Order #5662, (see below).
1 Product with this symbol may be used in Magnetic Resonance environment according to the conditions described in Directions for Use.

| Order Number | Stent O.D. (mm) | Proximal / Distal Flares O.D. (mm) | Stent Length (cm) | Covered Length (cm) | Catheter Diameter (Fr / mm) | Working Length (cm) | Packaging |
|---|---|---|---|---|---|---|---|
| M00516900 | 18 | 23 / 23 | 10.3 | 7 | 18.5 / 6.17 | 78 | Each |
| M00516910 | 18 | 23 / 23 | 12.3 | 9 | 18.5 / 6.17 | 78 | Each |
| M00516920 | 18 | 23 / 23 | 15.3 | 12 | 18.5 / 6.17 | 78 | Each |
| M00516930 | 23 | 28 / 28 | 10.5 | 7 | 18.5 / 6.17 | 78 | Each |
| M00516940 | 23 | 28 / 28 | 12.5 | 9 | 18.5 / 6.17 | 78 | Each |
| M00516950 | 23 | 28 / 28 | 15.5 | 12 | 18.5 / 6.17 | 78 | Each |
*Recommended Guidewire .038" Jagwire™ Guidewire, Order #5662, (see below).
1 Product with this symbol may be used in Magnetic Resonance environment according to the conditions described in Directions for Use.