Agile™
Esophageal Fully & Partially Covered Stent System
The Agile Esophageal Stent System, featuring a through-the-scope delivery system, is intended for maintaining esophageal luminal patency in esophageal strictures caused by intrinsic and/or extrinsic malignant tumors and occlusion of concurrent esophageal fistulas.
Product Details
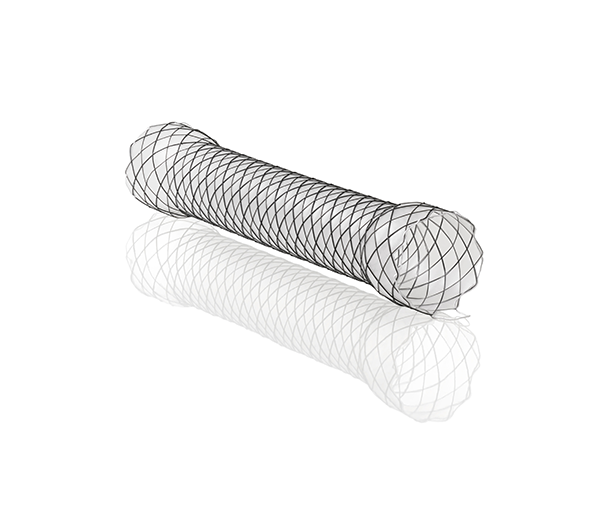
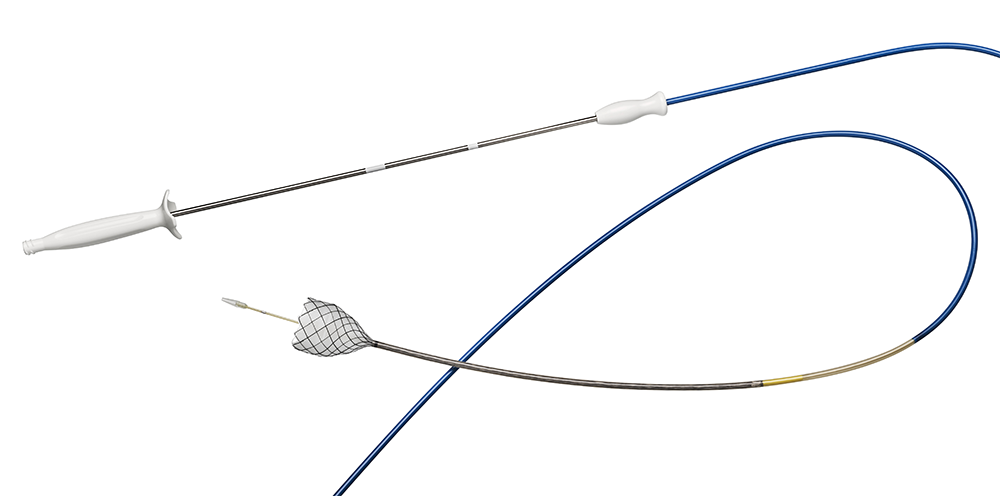
The Agile Esophageal Stent System offers a flexible and conformable self-expanding metal stent on a through the scope delivery system which enables direct visualization during deployment and may help minimize reliance on fluoroscopy.
- Offering sizing options including a 60mm length and 14mm diameter designed to meet a variety of clinical needs, all size configurations are offered in fully covered and partially covered options.
- Braided dual gauge nitinol wire provides an implant that is more flexible and conformable, as compared to WallFlexTM*.
- Tissue In-Growth Prevention—The silicone covering extends the entire length of the Fully Covered Agile Esophageal Stent and is designed to address tumor ingrowth.
Delivery System
Through-the-Scope design
- Provides direct visualization to aid physicians in stent placement and help minimize fluoroscopic dependence.
- Designed to offer flexibility and trackability to enhance physician control.
Low profile delivery system
Compatible with a 3.7mm working channel, designed to navigate through tight strictures.
5 radiopaque markers
Markers signal the ends of the stent, the midpoint of the stent, and the point at which the stent can no longer be recaptured, aiding in deployment. The fifth marker at the leading end of the exterior tube indicates how far the stent has been deployed.
Reconstrainability
In the event that the stent is not exactly where a physician intends it to be, the stent can be reconstrained up to 2x and redeployed.
Ordering Information
| Order Number | GTIN | Stent O.D. (mm) | Proximal/Distal Flares O.D. (mm) | Stent Length (cm) | Delivery System Diameter (Fr) |
|---|---|---|---|---|---|
| M00517200 | 08714729972907 | 14 | 19 | 6.2 | 10.5 |
| M00517210 | 08714729972914 | 14 | 19 | 10.2 | 10.5 |
| M00517220 | 08714729972921 | 14 | 19 | 11.9 | 10.5 |
| M00517230 | 08714729972938 | 14 | 19 | 14.8 | 10.5 |
| M00517240 | 08714729972945 | 18 | 23 | 5.9 | 10.5 |
| M00517250 | 08714729972952 | 18 | 23 | 9.7 | 10.5 |
| M00517260 | 08714729982969 | 18 | 23 | 11.9 | 10.5 |
| M00517270 | 08714729972976 | 18 | 23 | 14.9 | 10.5 |
| Order Number | GTIN | Stent O.D. (mm) | Proximal/Distal Flares O.D. (mm) | Stent Length (cm) | Delivery System Diameter (Fr) |
|---|---|---|---|---|---|
| M00517710 | 00191506006587 | 23 | 28 | 6.2 | 18.5 |
| M00517720 | 00191506006594 | 23 | 28 | 10.1 | 18.5 |
| M00517730 | 00191506006600 | 23 | 28 | 12.0 | 18.5 |
| M00517740 | 00191506006617 | 23 | 28 | 15.0 | 18.5 |
| Order Number | GTIN | Stent O.D. (mm) | Proximal/Distal Flares O.D. (mm) | Stent Length (cm) | Delivery System Diameter (Fr) |
|---|---|---|---|---|---|
| M00517400 | 08714729973027 | 14 | 19 | 6.2 | 10.5 |
| M00517410 | 08714729973034 | 14 | 19 | 10.2 | 10.5 |
| M00517420 | 08714729973041 | 14 | 19 | 11.9 | 10.5 |
| M00517430 | 08714729973058 | 14 | 19 | 14.8 | 10.5 |
| M00517440 | 08714729973065 | 18 | 23 | 5.9 | 10.5 |
| M00517450 | 08714729973072 | 18 | 23 | 9.7 | 10.5 |
| M00517460 | 08714729973089 | 18 | 23 | 11.9 | 10.5 |
| M00517470 | 08714729973096 | 18 | 23 | 14.9 | 10.5 |
| Order Number | GTIN | Stent O.D. (mm) | Proximal/Distal Flares O.D. (mm) | Stent Length (cm) | Delivery System Diameter (Fr) |
|---|---|---|---|---|---|
| M00517750 | 00191506006624 | 23 | 28 | 6.2 | 18.5 |
| M00517760 | 00191506006631 | 23 | 28 | 10.1 | 18.5 |
| M00517770 | 00191506006648 | 23 | 28 | 12.0 | 18.5 |
| M00517780 | 00191506006655 | 23 | 28 | 15.0 | 18.5 |
Reimbursement
Physician & Patient Resources
Case Studies
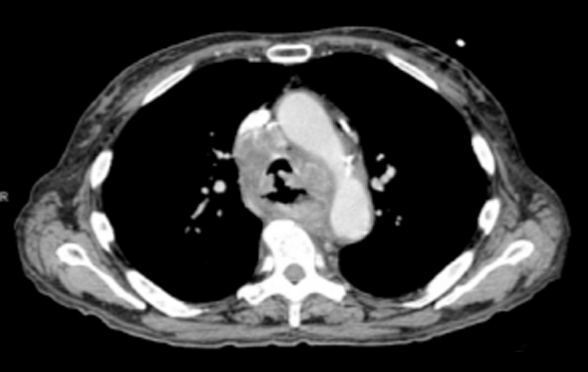
Stenting of Tracheo-Esophageal FistulaUnder Direct Visualization
Dr. Moremen discusses a case on stenting of trachea-esophageal fistula under direct visualization.