LATITUDE Link™ Data Management System
Seamless Data Integration
The days of having to print reports from a Boston Scientific cardiac rhythm management programmer, then scan and upload them to a patient’s chart are over! LATITUDE Link simplifies this workflow by enabling seamless transfer of programmer data and reports to an electronic medical record (EMR) system.
Key Resources
Product Details
The LATITUDE Link Data Management System is designed to easily move data and reports from a Boston Scientific programmer to the clinic EMR. Once data and reports are transferred via Bluetooth™, USB flash drive, or microSD card to a LATITUDE Link-enabled PC, LATITUDE Link uses the patient’s unique information – model and serial number of their implanted device, and their date of birth – to automatically save the data and reports in the patient’s EMR. If multiple reports are transferred, LATITUDE Link will combine these reports into a single PDF before saving the report, simplifying the review of the patient’s EMR data.
Benefits of LATITUDE Link:
- Eliminates printing and scanning
- Reduces possible human error
- Provides complete documentation in EMR
LATITUDE Link™ Data Management System Installation
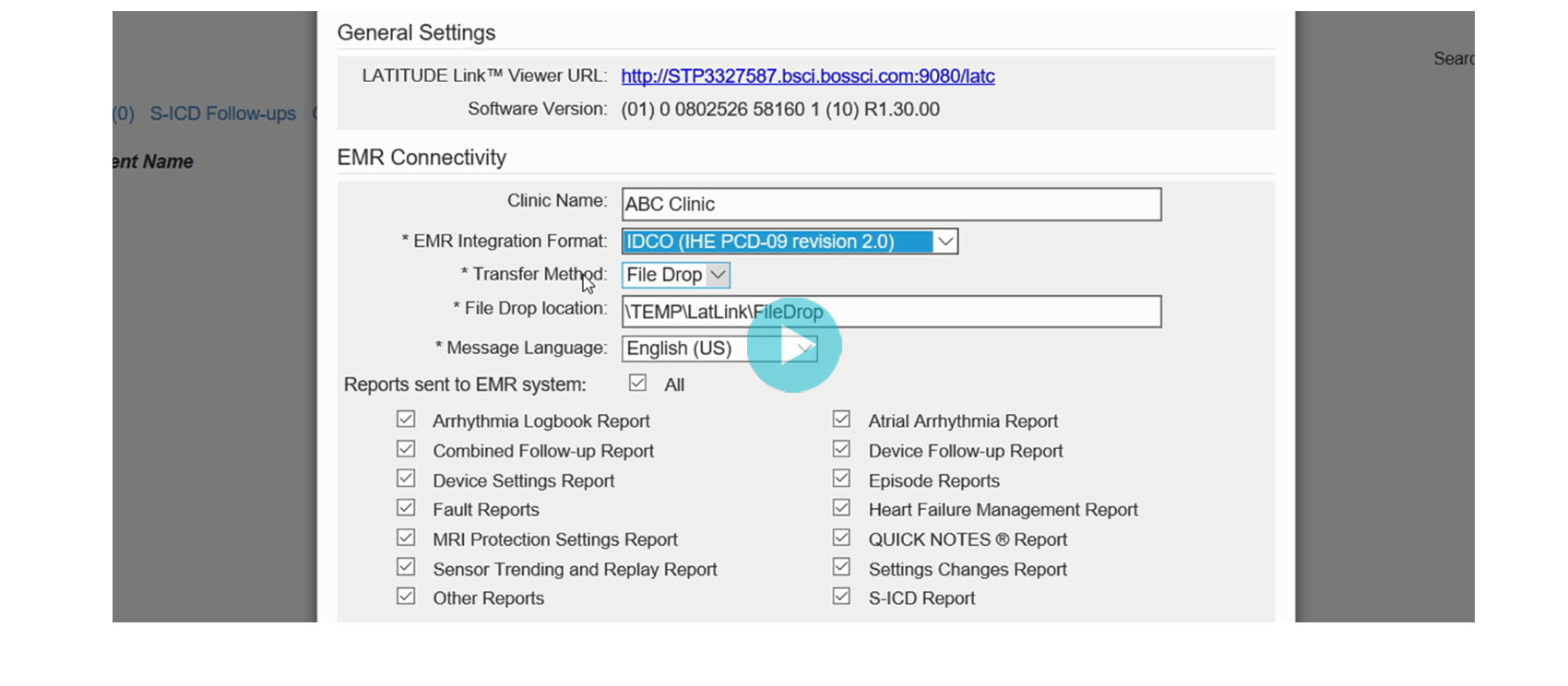
Downloads
Ordering Information
Reimbursement
Installation Manuals
Heart Connect
Find out how to share programmer screen information in real time using the Heart Connect™ System.

Ownership of CRM Programmers, LATITUDE Consult™ and Heart Connect™ Systems
In some cases, Boston Scientific provides CRM Programmers, LATITUDE Consult Systems, and/or Heart Connect Systems for temporary use by hospitals and clinics. Boston Scientific does not sell or transfer title to any of this equipment within the United States, and any CRM programmers (including the model 3120, 3200, and 3300 programmers), LATITUDE Consult Communicators, and Heart Connect Systems in the U.S. are owned by Boston Scientific. If you need to return one of these devices to Boston Scientific, please contact 1-800-CARDIAC (227-3422) for instructions. If you have purchased or been given one of these devices by anyone other than Boston Scientific, please be advised that you do not have proper legal title to the equipment and that legal ownership remains with Boston Scientific. Boston Scientific reserves all rights to recover any equipment that has been illegally transferred or sold to third parties and to pursue legal action for such improper transfer.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. This material not intended for use in France. 2019 Copyright © Boston Scientific Corporation or its affiliates. All rights reserved.

