Aegis™
One-Hour Indicator
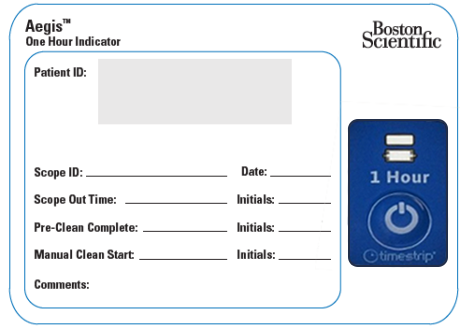
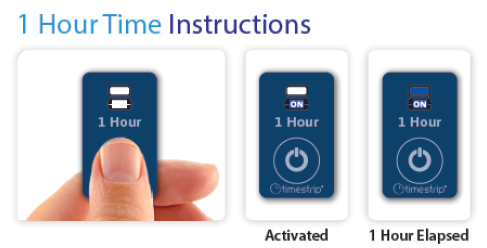
Aegis One-Hour Indicator provides a clear visual indicator and peel-off label as a reminder of the one-hour time constraint to help nursing and reprocessing staff comply with the endoscope manufacturer's reprocessing requirements.
The Aegis One-Hour Indicator is designed to adhere to CinchPad™ and other systems used to transport contaminated endoscopes. The peelable label can help users to conveniently track and document endoscope reprocessing timelines.
Step-by-step Process
Industry Information
A process must be in place to record the procedure end time and the manual cleaning start time. This process will allow personnel to determine whether routine reprocessing within the manufacturer’s recommended time frame is achievable and, if not, to implement the manufacturer’s procedures for delayed reprocessing (CDC, 2017). Reprocessing personnel must refer to the manufacturer’s recommendations for delayed re-cleaning and reprocessing.
Ordering Information
Order Number | Description | Units |
M00501991 | Aegis™ One-Hour Indicator | Box 100 |