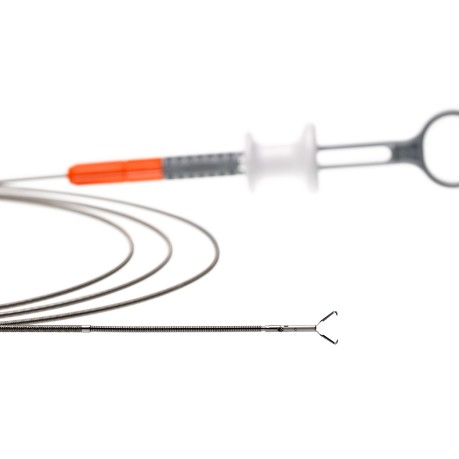
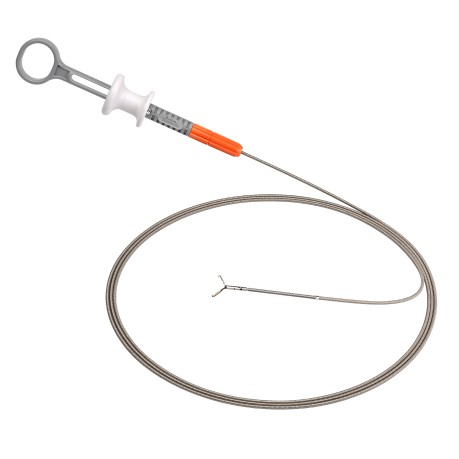
MANTIS Clip (abbreviated as MANTIS) is purpose-built for closing large defects¹ in the GI tract. With proprietary TruGrip™ anchor prongs, MANTIS is designed to deliver tissue span and apposition capabilities, enabling an approach to closing defects.*2,3
Product Details
At last, a device designed for large defect closure.1
Potential to improve efficiency
A single MANTIS has potential to do the job of multiple conventional clips.5
Designed to limit slipping
TruGrip™ anchor prongs are designed to allow users to securely grasp healthy tissue, which can limit slippage during mobilization.2,4
Precision placement features
Enable faster, more accurate placement through one-to-one physician-controlled rotation.6
Enable a three-step approach
Achieve tissue apposition and closure through the 3-step approach to closing defects: Anchor, Mobilize, Close.2,3
Ordering Information

| Order Number | Description | Working Length (cm) | Minimum Working Channel (mm) | Unit |
|---|---|---|---|---|
| M00521420 | MANTIS Clip | 235 cm | 2.8 mm | Box 1 |
| M00521421 | MANTIS Clip | 235 cm | 2.8 mm | Box 10 |
| M00521422 | MANTIS Clip | 235 cm | 2.8 mm | Box 20 |
| M00521423 | MANTIS Clip | 235 cm | 2.8 mm | Box 40 |
Reimbursement
*Please refer to Instruction for Use regarding Indications, contraindications and warnings
1. Liaquat, H., Rohn, E., & Rex, D. K. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: Experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointestinal Endoscopy, 2013, 77(3): ,401–407. https://doi.org/10.1016/j.gie.2012.10.024
2. Boston Scientific conducted a bench study to gather feedback from 16 paid physicians on the use of commercially representative MANTIS™ Clips on excised porcine stomach model and synthetic tissue model. Data on file. The objective of the study was to evaluate MANITS Clip’s tissue apposition and mobilization design features as compared against Boston Scientific Resolution™ 360 Clips, and other competitive clips. The bench study was performed with minimal training via some preparation in the ex-vivo model. Bench testing results may not necessarily be indicative of clinical performance.
3. The pre-clinical testing was performed by BSC. Data on file. Sixteen paid physicians used MANTIS with anchor, mobilize and close approach on porcine model. Pre-clinical study may not necessarily be indicative of clinical performance.
4. The testing was performed by BSC. Data on file. Bench testing results may not necessarily be indicative of clinical performance.
5. Boston Scientific conducted a bench study to gather feedback from 16 paid physicians on the use of commercially representative MANTIS™ Clips on excised porcine stomach model and synthetic tissue model. Data on file. The objective of the study was to evaluate MANTIS Clip’s tissue apposition and mobilization design features as compared against Boston Scientific Resolution™ 360 Clips, and other competitive clips. The bench study was performed with minimal training via some preparation in the ex-vivo model. Bench testing results may not necessarily be indicative of clinical performance. Each physician conducted one closure per product brand. The data showed that the average time and clip quantity to close the defects when using the MANTIS Clip was less than the combined average time (reduced by 4 mins 45 seconds) and clip quantity (reduced by 4) for the Resolution 360 and other competitive devices. Bench testing results may not necessarily be indicative of clinical performance.
6. Wang TJ, Aihara H, Thompson AC, Schulman AR, Thompson CC, Ryou M. Choosing the right through-the-scope clip: a rigorous comparison of rotatability, whip, open/close precision, and closure strength (with videos). Gastrointest Endosc. 2019;89(1):77-86.e1. doi:10.1016/j.gie.2018.07.025. MANTIS is built on the Resolution 360 Clip platform referenced in the study. CAUTION: U.S. Federal law restricts this device to sale by or on the order of a physician.