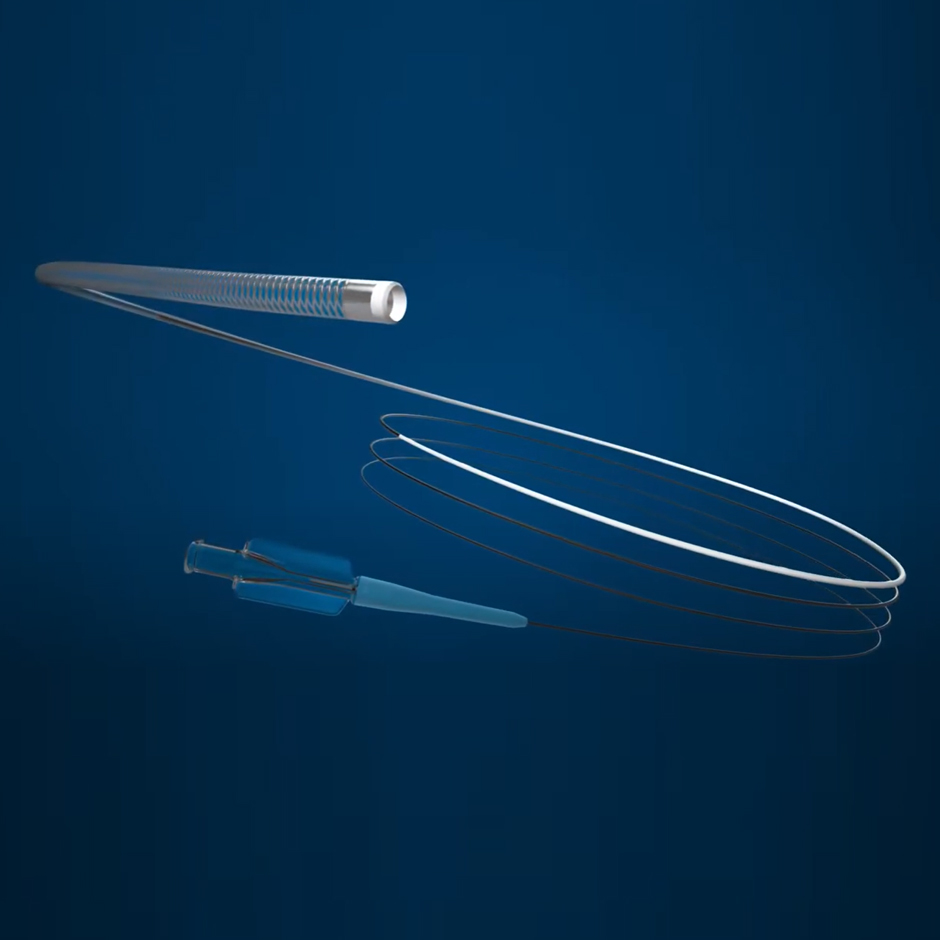
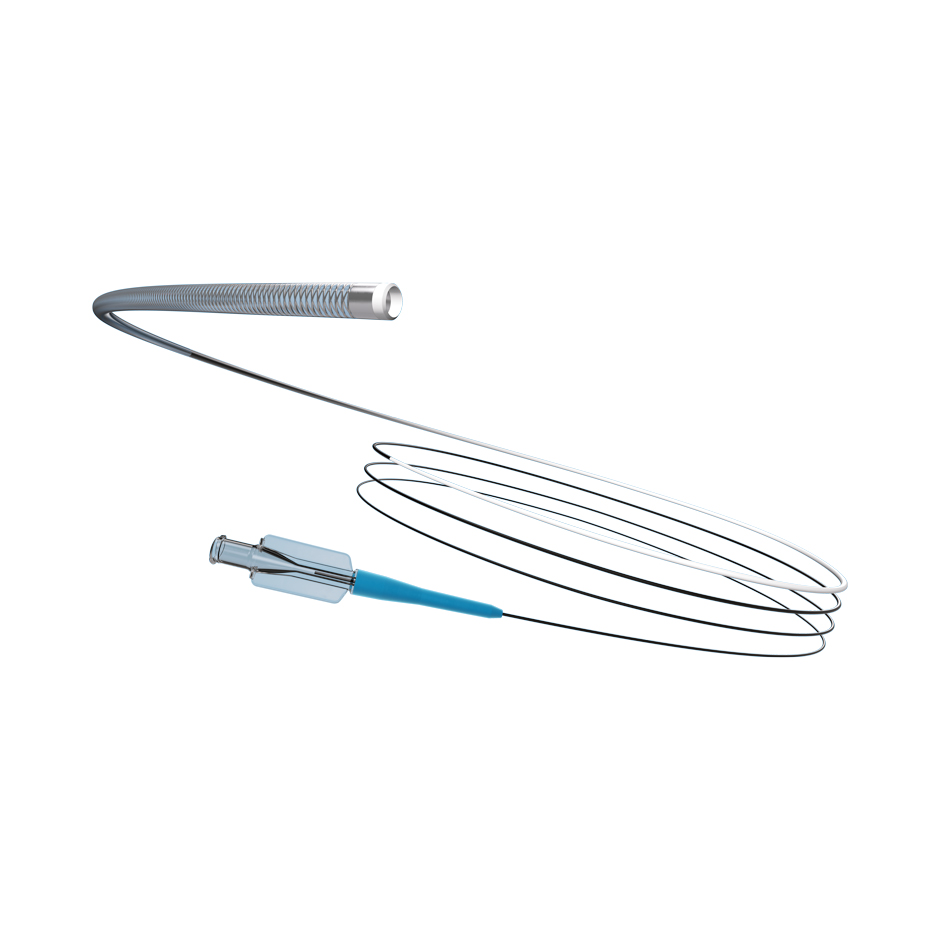
TRUSELECTTM
Microcatheter
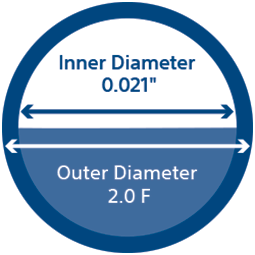
The unique 2.0 F design and 0.021” inner diameter lets you go further, without sacrificing the superior flow rates and embolic compatibility you need.
Product Details
Selective Embolization You Control
TruSelect™ Microcatheters are designed for interventionalists. Engineered with an 0.021” inner diameter within a 2.0 F design and the first 175 cm length, with a balance of pushability and flexibility providing enhanced trackability, TruSelect Microcatheters allow interventionalists to get closer to intended targets without sacrificing flow rates or embolic choices.
Case Studies
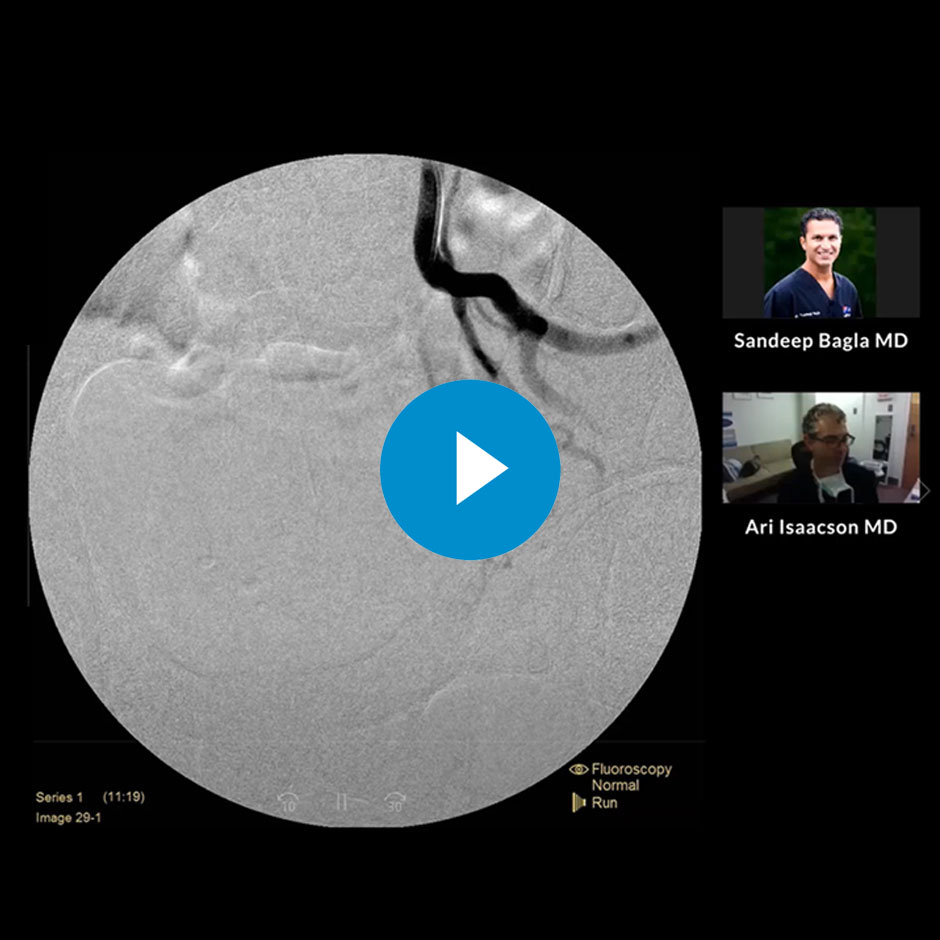
TruSelect Microcatheter Live Case Recording
See Sandeep Bagla, MD and Ari Isaacson, MD perform and discuss using a TruSelect Microcatheter for a Prostate Artery Embolization (PAE) procedure from radial access.
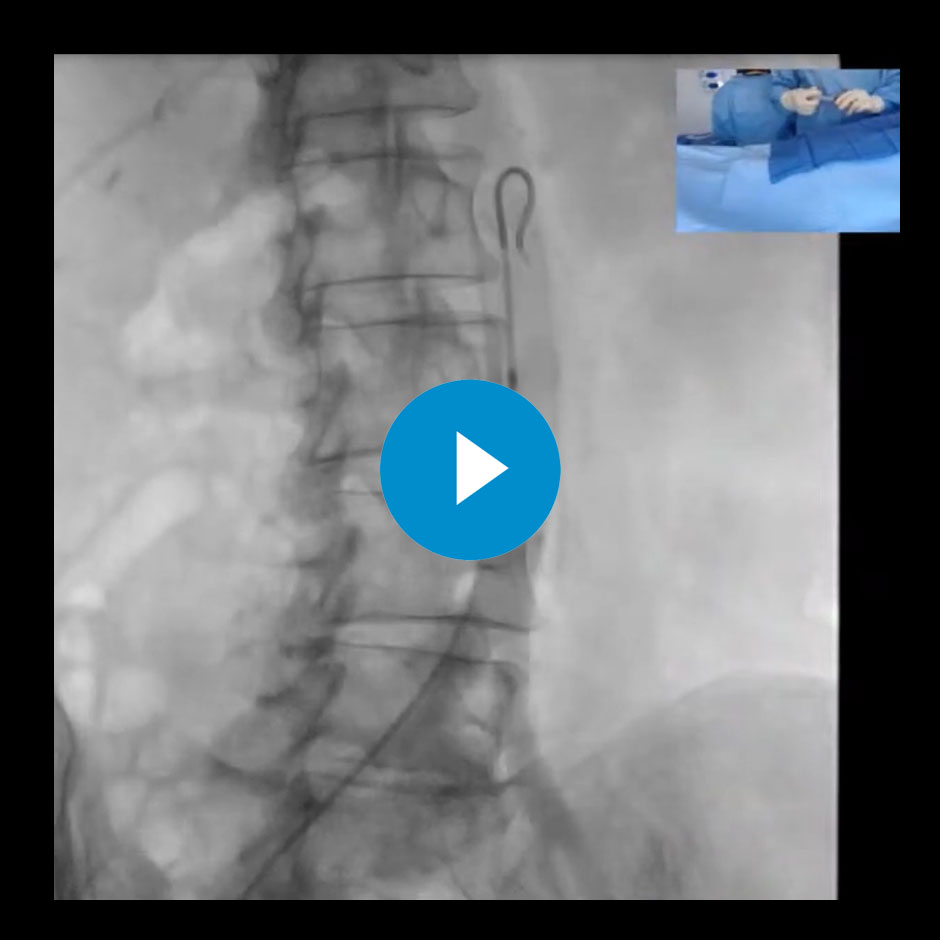
Hemorrhoid Embolization Live Case Recording
Watch Sandeep Bagla, MD and Ari Isaacson, MD perform and discuss doing a hemorrhoid embolization using a TruSelect Microcatheter, Interlock Detachable Coils, and Bead Block Embolic Agents.
Ordering Information
| UPN | Usable Length (cm) |
Proximal/distal O.D. (F) |
Inner Diameter (in) |
Tip Shape | Max Burst Rating (PSI) |
| M001394101050 | 105 | 2.8/2.0 | 0.021" | Straight | 800 |
| M001394101300 | 130 | Straight | |||
| M001394101550 | 155 | Straight | |||
| M001394101750 | 175 | Straight | |||
| M001394111050 | 105 | Bern | |||
| M001394111300 | 130 | Bern | |||
| M001394111550 | 155 | Bern | |||
| M001394111750 | 175 | Bern |
Reimbursement
C-codes are used to report devices used in combination with device-related procedures for hospital outpatient services. The applicable C-code to report the use of TruSelect™ is C1887, defined as “Catheter, guiding (may include infusion or perfusion capability).”
Note: Boston Scientific is not responsible for the correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.
Resources
PDF (1.2 MB)
PDF (8.9 MB)
Microcatheters and Guidewires Brochure
PDF (1.7 MB)