LC Bead LUMI™
Radiopaque Embolic Bead
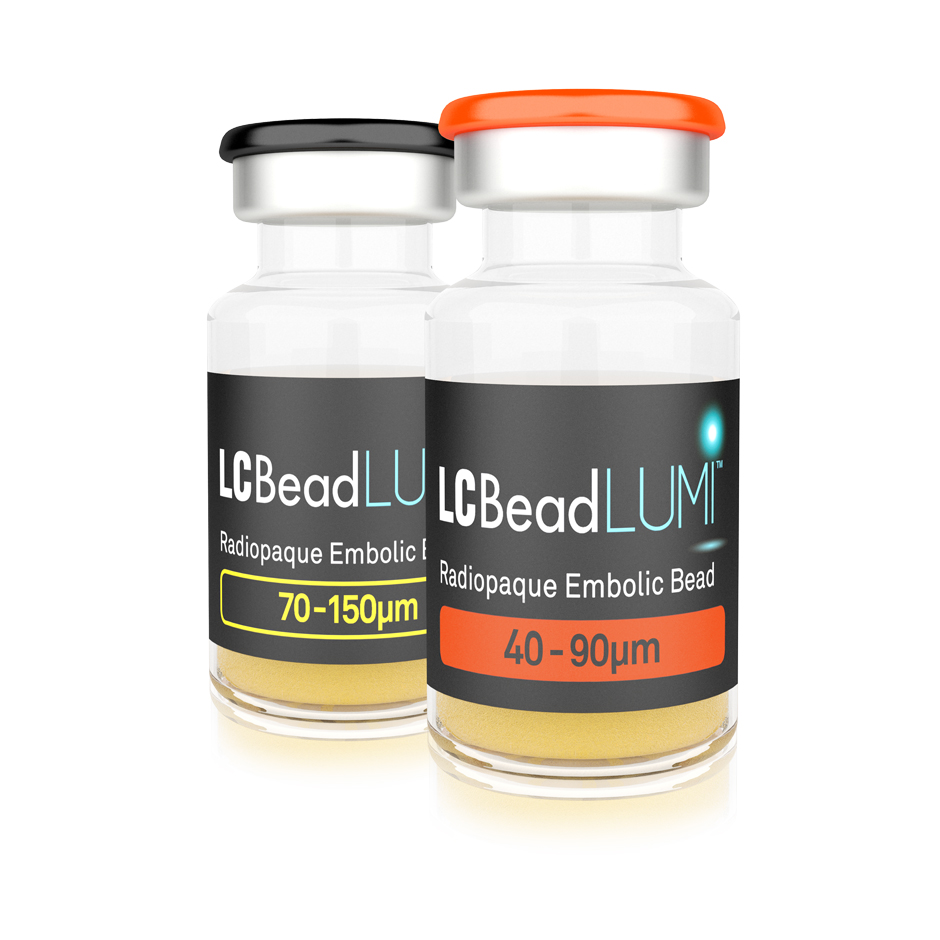
LC Bead LUMI is the world’s first microsphere offering inherent, long-term radiopacity combined with the trusted performance of LC Bead
Key Resources
Product Description
LC Bead LUMI™ — See More. Treat Smarter.
VISION
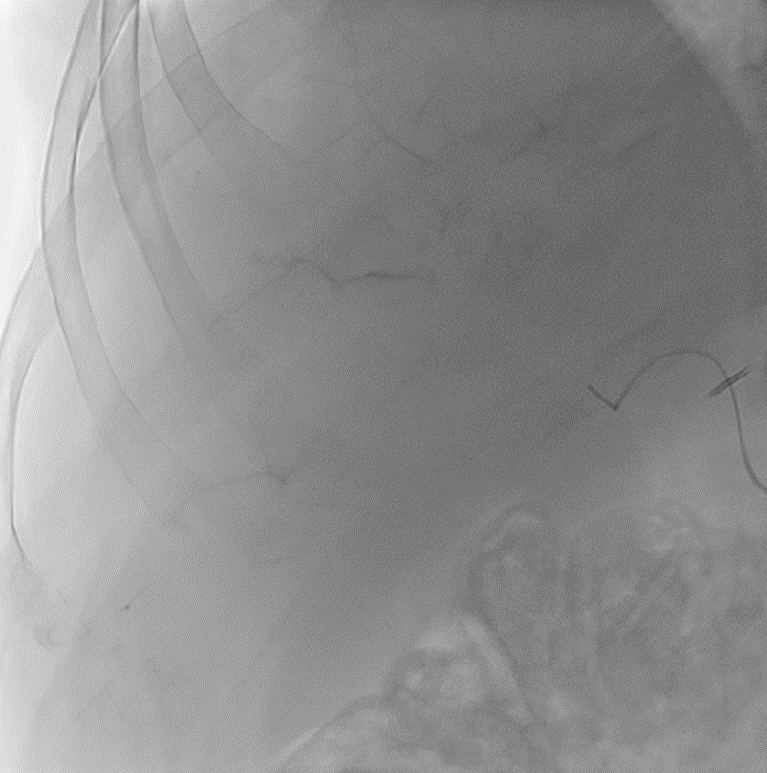
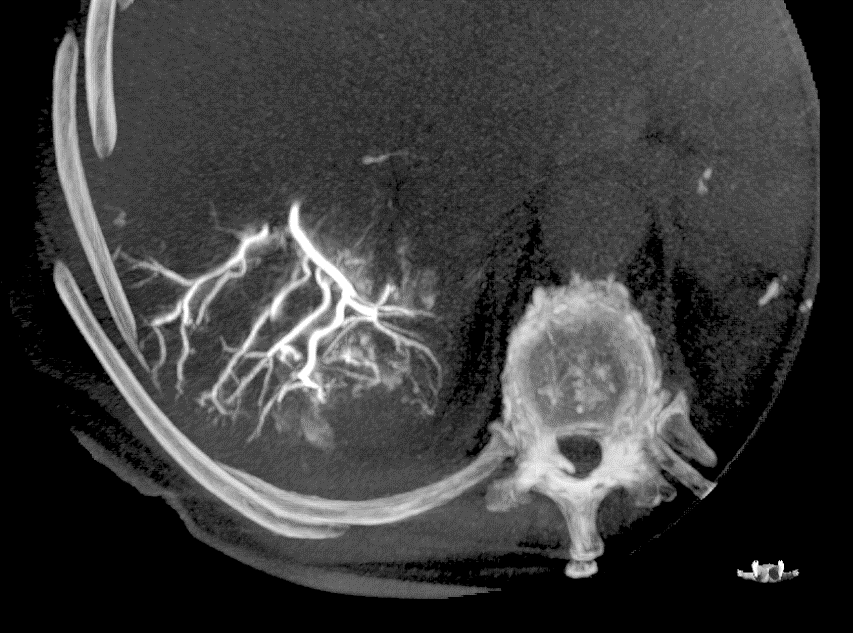
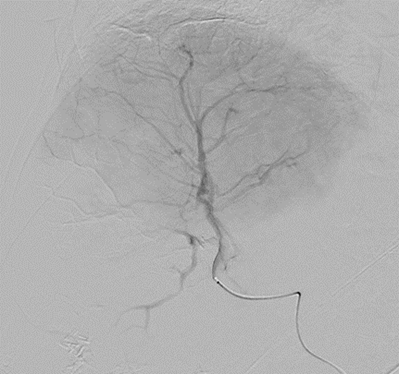
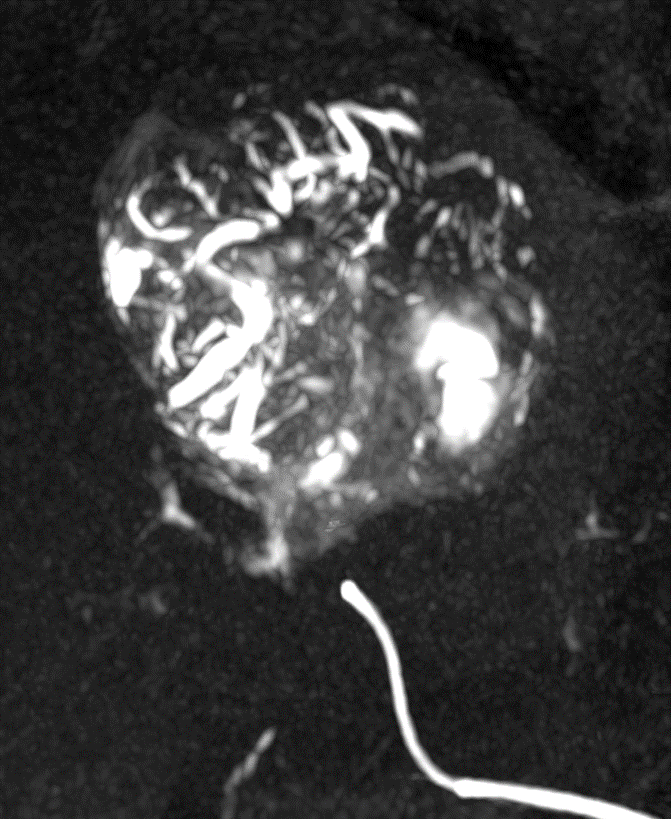
With LC Bead LUMI™, you are empowered to see the bead during and after treatment. During LUMI embolization — see precisely where LC Bead LUMI™ need to be delivered1.
Fluoroscopy Single shot; images courtesy NIH with permission*.
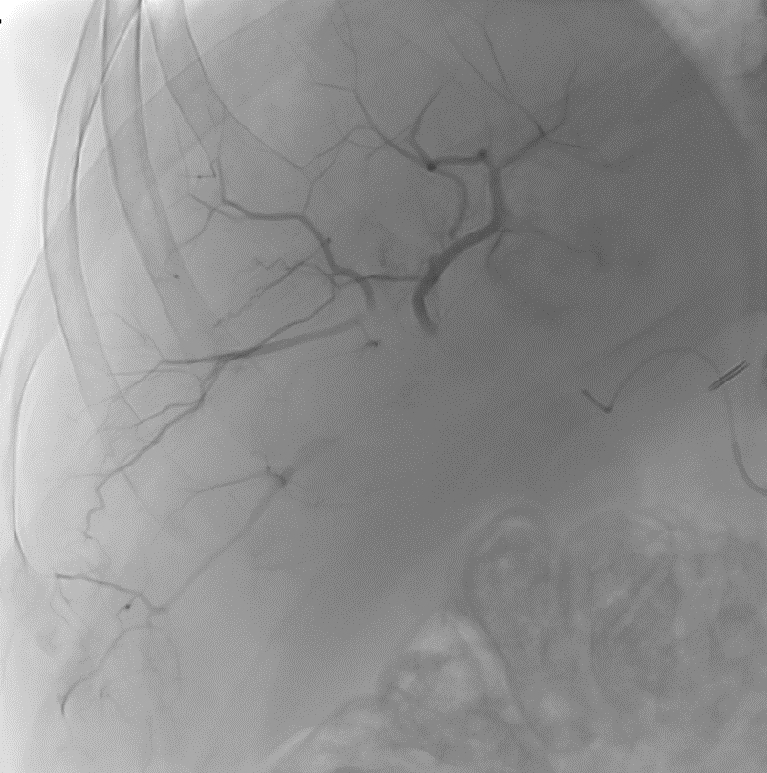
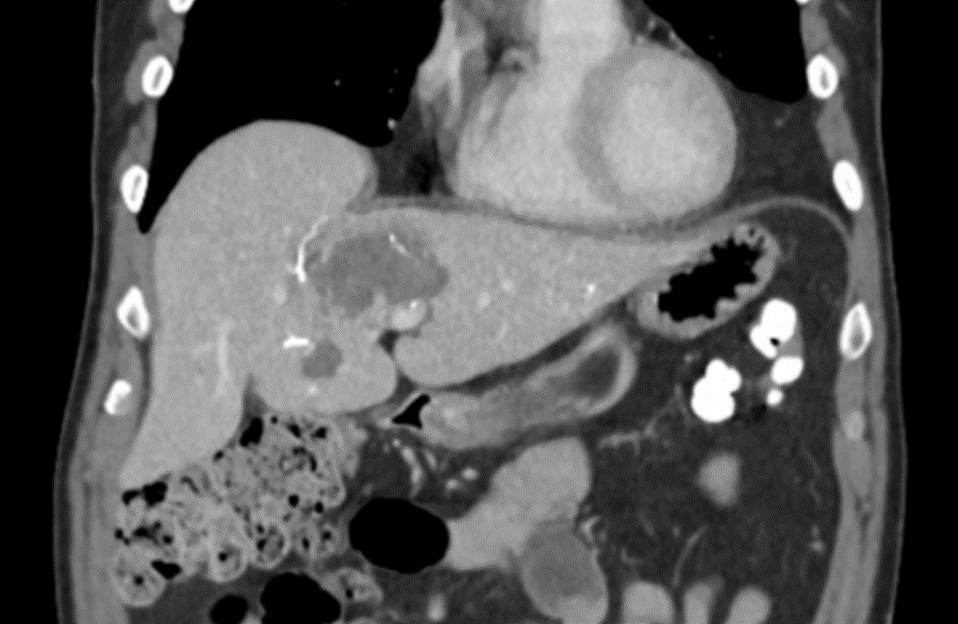
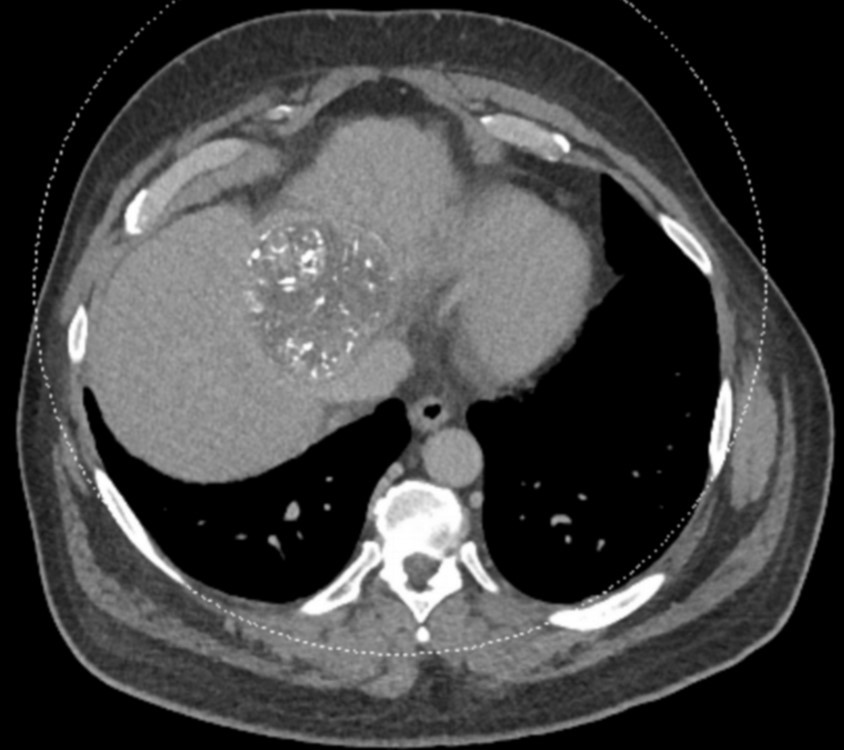
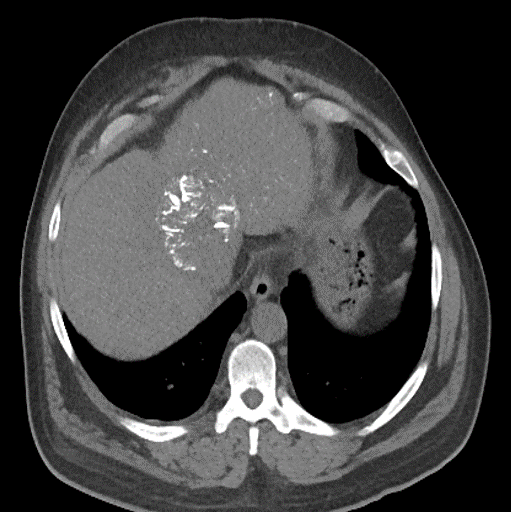
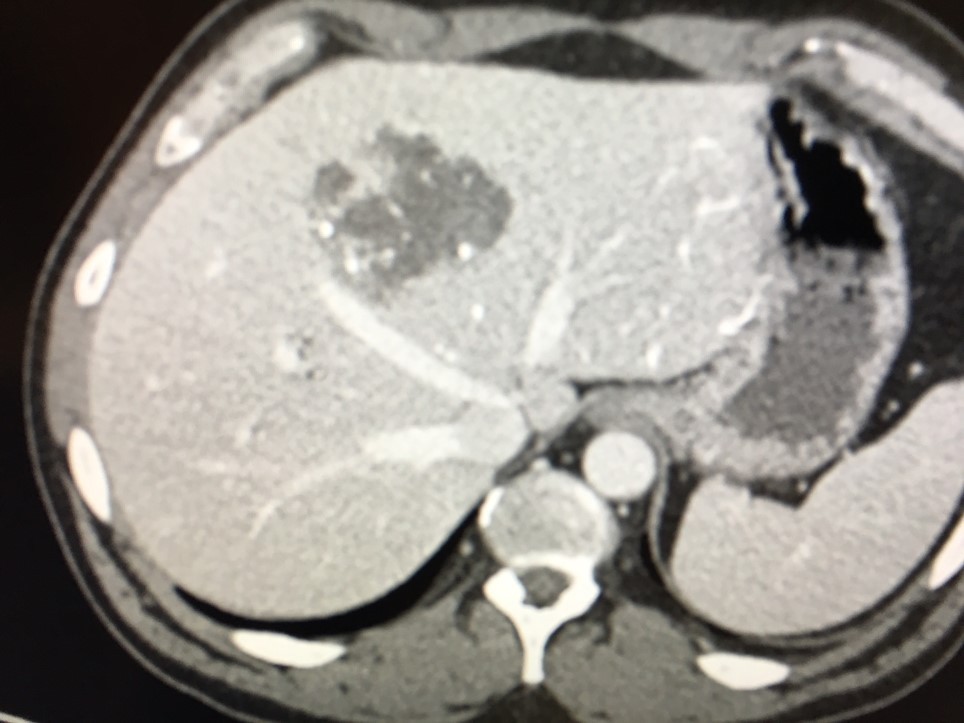
After LUMI embolization — with lasting radiopacity, LC Bead LUMI™ continues to be visible in follow-up scans1.
ACC: adenoid cystic carcinoma; CBCT MIP: Cone-beam computed tomography maximum intensity projection; DSA: digital subtraction angiography; HCC: hepatocellular carcinoma; MDCT: multidetector computed tomography; NET: neuroendocrine tumor. Images courtesy of NIH with permission*.
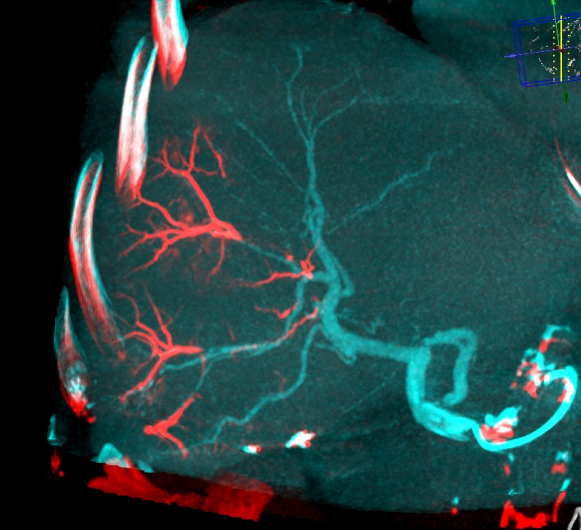
LC Bead LUMI™ offers you a new level of control with real-time feedback both during and after embolization.
PRECISION
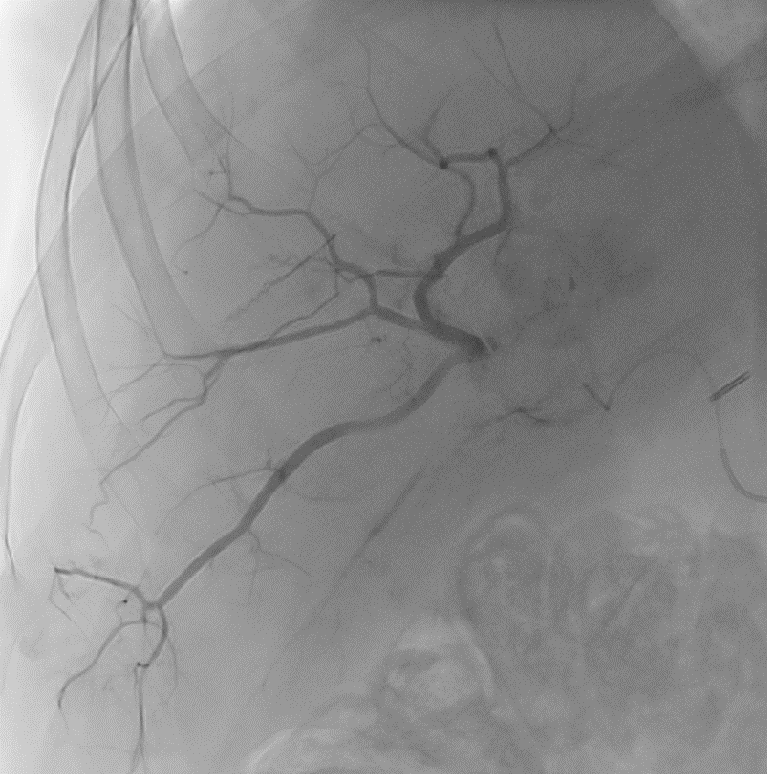
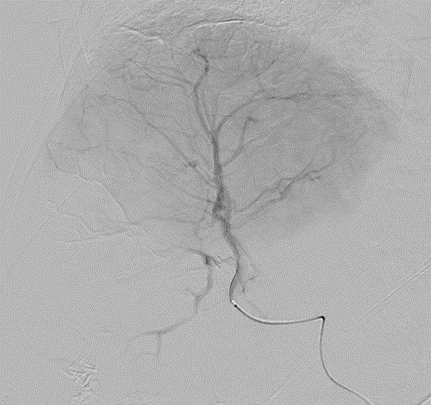
LC Bead LUMI™ is designed to offer a more precise and controlled procedure than current techniques.
CBCT MIP: Cone-beam computed tomography maximum intensity projection; DSA: digital subtraction angiography; HCC: hepatocellular carcinoma. Images courtesy of NIH with permission*.
LC Bead LUMI™ enables real-time adjustment during the whole procedure1.
ASSURANCE
LC Bead LUMI™ offers the precision and control you need to:
Product Specifications and Sizes
LC Bead LUMI™ are precisely calibrated, radiopaque, biocompatible, nonresorbable hydrogel beads. The beads are produced from polyvinyl alcohol and contain a covalently bound radiopaque moiety.
LC Bead LUMI™ are manufactured to be inherently radiopaque and visible under imaging (Computed Tomography [CT], Cone Beam Computed Tomography [CBCT] and Fluoroscopy). LC Bead LUMI™ are available in two size ranges.
Presentation
- LC Bead LUMI™ are offered in a prefilled 10ml glass vial, stopper sealed by an aluminum cap with a color-coded lid.
- Each vial contains approximately 2ml of product in sterile phosphate buffered saline. The total volume of LC Bead LUMI™ and sterile physiological saline is approximately 8ml.
- Each package contains a sterile 20mm ViaLok™ Vented Vial Access Device (Yukon Medical LLC, 4021 Stirrup Creek, Durham, NC USA) for removal of LC Bead LUMI™ from the vial.
- Each vial of LC Bead LUMI™ is intended for single patient use only. Do not re-sterilize. Discard any unused material.
Recommended Catheters and Contrast Agents for application with LC Bead LUMI
Catheters
Once prepared, LC Bead LUMI has been tested and shown to be successfully delivered using the combinations of microspheres size and microcatheters shown in the table below.
| Product Size Range of LC Bead LUMI | Recommended Catheter (Internal Diameter) |
| 40-90μm (40-100μm)* | ≥2.0Fr (0.483mm/0.019in) |
| 70-150μm (70-170μm)* | ≥2.0Fr (0.483mm/0.019in) |
Contrast Agents
LC Bead LUMI must be suspended in soluble iodinated contrast (a minimum of 20ml per vial) to achieve effective delivery through microcatheters and to aid visualization during administration. Only pure contrast (i.e. undiluted) should be used. The LC Bead LUMI IFU recommends using contrast agents Omnipaque 350 (Iohexol 350) and Iomeron 400 (Iomeprol 400). Based on post-approval early experience, please note that Visipaque provides an even better (long and stable) suspension. LC Bead LUMI is visible on X-ray as the beads accumulate in the vessel and the contrast clears.
- Other contrast agents have not been tested in conjunction with LC Bead LUMI.
- Optiray 350, Oxilan 350, and Isovue 370 have not been tested for the 300-500μmm size range.
- Isovue 300 (Iopamidol) has been tested and is not recommended for use due to the inadequate suspension times. Contrast agents of a similar or lower viscosity at 20° C should not be used with LC Bead LUMI.
Radiopacity and Visualization
LC Bead LUMI is visible under x-ray imaging as they accumulate in the embolized vessels. It can be directly visualized as the delivery contrast agent washes out. Best visualization is achieved after administration using X-ray single shot technique (also referred to as X-ray snap shot). Cone beam CT (CBCT) can also be used to visualize the beads accumulated in the vessels with multi-planar reconstructions providing 3D spatial bead location and vessel connectivity.
LC Bead LUMI is easily visualized with CT. Early experience images from various different tumor types, show discrete embolized vessels where LC Bead LUMI is present with no significant streak artifacts nor masking of adjacent tissue unlike commonly seen following lipiodol-containing treatments. If contrast enhanced CT images are desired, obtaining a non-contrast image may be helpful to discriminate LC Bead LUMI from contrast enhancement.
The radiopacity of LC Bead LUMI does not degrade over time, so the beads will continue to be visible at follow-up.