The S-ICD Pacing Risk Share Program
Why a Risk Share Program?
The S-ICD Pacing Risk Share Program is being offered by Boston Scientific so that we may share in the low risk of a patient developing a need for bradycardia pacing or anti-tachycardia pacing (ATP) after the implant of an S-ICD.
Through this program, physicians can be confident they are offering patients protection from sudden cardiac death without the risks associated with transvenous leads while also receiving support from Boston Scientific in the unlikely event that a patient’s pacing need changes.
How many ICD recipients benefit from ATP?
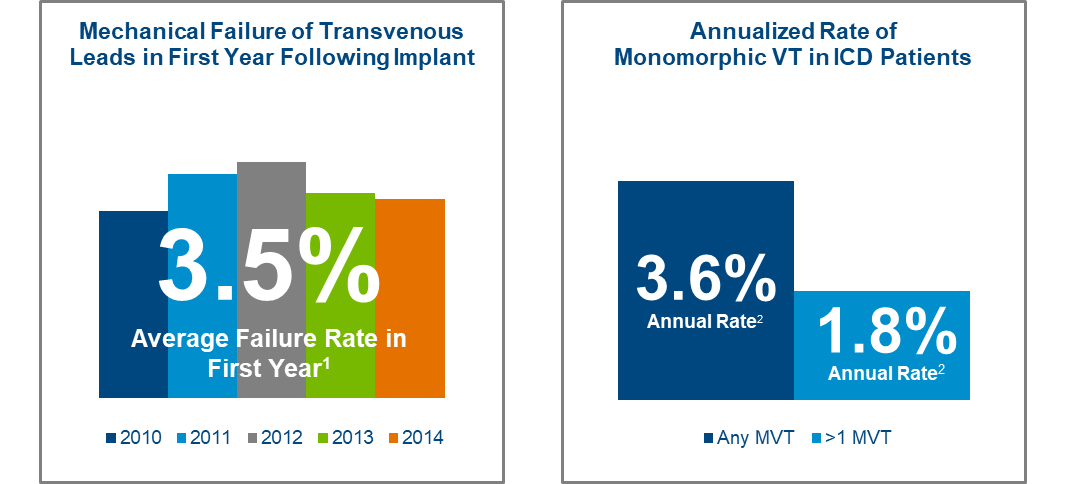
Data from SCD-HeFT demonstrated that the percent of patients who experience at least one episode of monomorphic VT (MVT) is approximately 3.6% per year. Only half of those patients, or 1.8% of ICD recipients, have multiple MVTs per year.2
Although ATP may provide a potential benefit to a small subset of patients, all patients implanted with a transvenous ICD are exposed to the risk of serious complications associated with transvenous leads. Data published in 2018 from the OptumLabs Data Warehouse, which include diagnosis, physician and procedure codes, and claims from patient hospitalizations, determined the average annual rate of mechanical lead failure in the first year following a transvenous ICD implant was 3.5%.1
How many ICD recipients develop a need for bradycardia pacing?
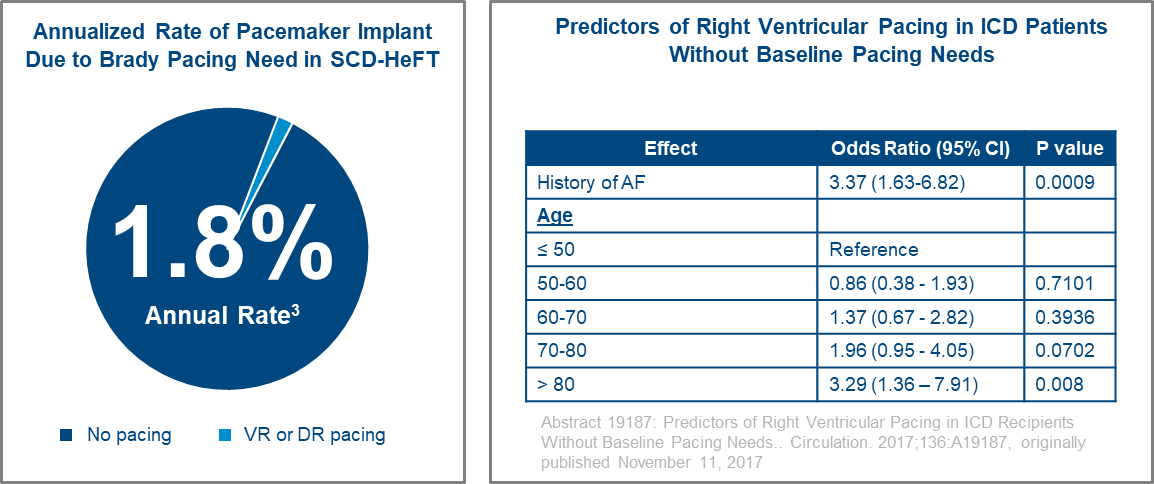
Data from SCD-HeFT demonstrated a very low annualized rate of 1.8% of single chamber ICD patients requiring a pacemaker implant during the multi-year study.3 Additionally, a study focused on the predictors of right ventricular pacing in single chamber ICD patients without baseline pacing needs found that the primary predictors for the development of need for bradycardia pacing are history of AF and being over age 80.4
What’s the bottom line?
When so few ICD recipients experience MVTs or develop a need for brady pacing following their ICD implant, why assume the risk of exposing all patients to the serious complications associated with transvenous leads? With the S-ICD Pacing Risk Share Program, Boston Scientific now has those few patients covered and the patient’s vasculature will be preserved until additional therapy is needed.
Program Overview
Program Participation
All patients who are implanted with Boston Scientific’s S-ICD in the United States on or after February 1, 2019 are automatically covered by the Risk Share Program if both the S-ICD and subsequent transvenous system implants were performed in the United States.
There is no financial commitment required from customers to participate in the program and there is no need to sign a new contract.
Boston Scientific reserves the right to amend or cancel this program at its sole discretion at any time.
Program Eligibility Requirements
- The S-ICD device has a registered implant date on or after February 1, 2019 and the implant was performed in the United States.
- The patient must have developed a need for bradycardia pacing per established guidelines or a need for anti-tachycardia pacing per physician judgment within three (3) years of the registered S-ICD implant date.
- The patient must have received a subsequent Boston Scientific transvenous pacemaker or ICD system within three (3) years of the registered S-ICD implant date and the replacement implant was performed in the United States.
- The patient must not have had a previous claim submitted under the Risk Share Program.
- The hospital performing the subsequent device implant must submit a claim using the Credit Request Form within 30 days of the registered device implant date.
Submitting a Claim
Frequently Asked Questions
Q1: What is the eligibility period for the program?
Q2: Do explanted S-ICD devices need to be returned to Boston Scientific to receive the program discount?
Q3: Does the hospital need to sign a new contract with Boston Scientific to participate in the program?
Q4: Can the program discount be applied to non-Boston Scientific products?
Q5: Can the program discount be applied to Boston Scientific CRT products or another Boston Scientific S-ICD?
References
- Koneru, JN, Jones, PW, Hammill, EF, Wold, N, and Ellenbogen, KA, Risk Factors and Temporal Trends of Complications Associated With Transvenous Implantable Cardiac Defibrillator Leads. J Am Heart Assoc, 2018. 7(10)
- Who Should Receive the Subcutaneous Implanted Defibrillator? Jeanne E. Poole and Michael R. Gold. Circulation: Arrhythmia and Electrophysiology. 2013;6:1236-1245, originally published December 17, 2013
- Gillespie, S, Incidence of pacemaker implantation in SCD-HeFT: Are single-chamber ICDs enough in heart failure? Heart Rhythm, 2014. Vol. 11(No. 5 May Supplement): p. PO 01-32
- Abstract 19187: Predictors of Right Ventricular Pacing in ICD Recipients Without Baseline Pacing Needs. Circulation. 2017;136:A19187, originally published November 11, 2017