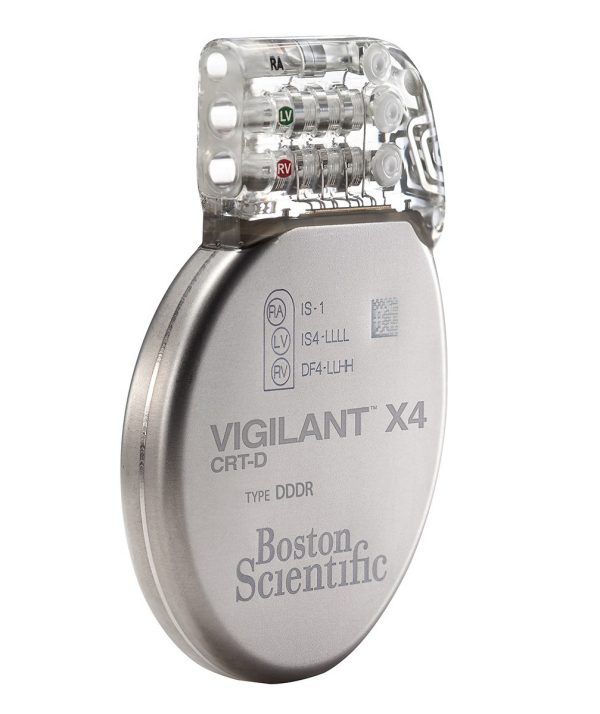
VIGILANT™ X4 CRT-D
Powered by EnduraLife™ Battery Technology
Model G247
The VIGILANT™ X4 CRT-D features EnduraLife™ Battery Technology and SmartCRT™ Technology, in addition to the HeartLogic™ Heart Failure Diagnostic and ImageReady™ MR-Conditional technology to help manage patient comorbidities.
Key Resources
Product Details


EnduraLife Battery Technology offers up to 13.3 years projected longevity with MultiSite Pacing turned on.**

Personalize CRT therapy with smart solutions that allow you to optimize where, when and how to pace.
*Boehmer JP, Hariharan R, Devecchi FG, et al. A Multisensor algorithm predicts heart failure events in patients with implanted devices: resultsfrom the MultiSENSE study. JACC Heart Fail. 2017 Mar;5(3):216-25.
** Assumes: 2.0V RA, LV-only, 2.0V LV, 700Ω, 15% A pacing, 100% LV pacing, No LATITUDE, No Respiratory Rate Sensor, No Heart Failure Sensor Suite.
†When conditions of use are met.
Mechanical Specifications
| Model | Type | Size (cm) (W x H x D) | Mass (g) | Volume (cc) | Connector Type (RA RV LV) | C-Code |
|---|---|---|---|---|---|---|
| G247 | X4 CRT-D | 5.37 x 8.18 x 0.99 | 73.8 | 32.5 | RA: IS-1; RV: DF4; LV: IS4 | C1882 |
Longevity Information
| Projected longevitya | Ventricular Chambers |
RA/RV | LV | LVbd | 500Ω with LATITUDE™b |
700Ω with LATITUDE™b |
700Ω no LATITUDE™, RS, or HFSSc |
|---|---|---|---|---|---|---|---|
| MultiSite Pacing Off | |||||||
| Typical programmed setting | BiV | 2.5 V | 3.0 V | Off | 9.7 | 10.5 | 11.3 |
| Maximum labeled longevity | LV-Only | 2.0 V / Off | 2.0 V | Off | 12.9 | 13.2 | 14.7 |
| MultiSite Pacing On | |||||||
| Typical programmed setting | BiV MSP | 2.5 V | 3.0 V | 3.0 V | 8.2 | 9.1 | 9.7 |
| Maximum labeled longevity | LV-Only MSP | 2.0 V / Off | 2.0 V | 2.0 V | 11.5 | 12.1 | 13.3 |
- Assumes 70 PPM LRL; DDDR mode; 0.4 ms Pulse Width (RA, RV, LV); sensors On, Heart Failure Sensor Suite On.
- Projected longevity is calculated assuming 2 maximum energy charging cycles per year, including automatic capacitor re-forms and therapeutic shocks. These calculations also assume 3-channel EGM Onset is on and that the pulse generator spends 3 months in Storage mode during shipping and storage.
a. Assumes ZIP telemetry use for 2 hours at implant and for 40 minutes annually for in-clinic follow-up checks.
b. Assumes standard use of the LATITUDE™ Communicator as follows: Daily Device Check on, quarterly scheduled remote follow ups, and other typical interrogations.
c. Assumes LATITUDE™ Communicator is not used, Respiratory Sensor is Off, and Heart Failure Sensor Suite is Off.
d. Applies to models with MultiSite Pacing (MSP).
Additional Longevity Information
- Boston Scientific devices have corporate warranties at 6 years in available geographies. Warranty information available at www.bostonscientific.com/warranty.
- Devices use Li/MnO2 chemistry.
- The Usable Battery Capacity is 1.9 Amp-hours (typical implant to battery capacity depleted).
- Shelf life is 2 years (before use by date).