RUNWAY™
Guide Catheter
High-performance design for exceptional support, strength and control in case after case.
Product Detail
Primary and Secondary Curve Firmness
- Provides outstanding back-up support
- Enhances pushability through tortuous anatomy
- Innovative polymer specially designed to enhance curve retention
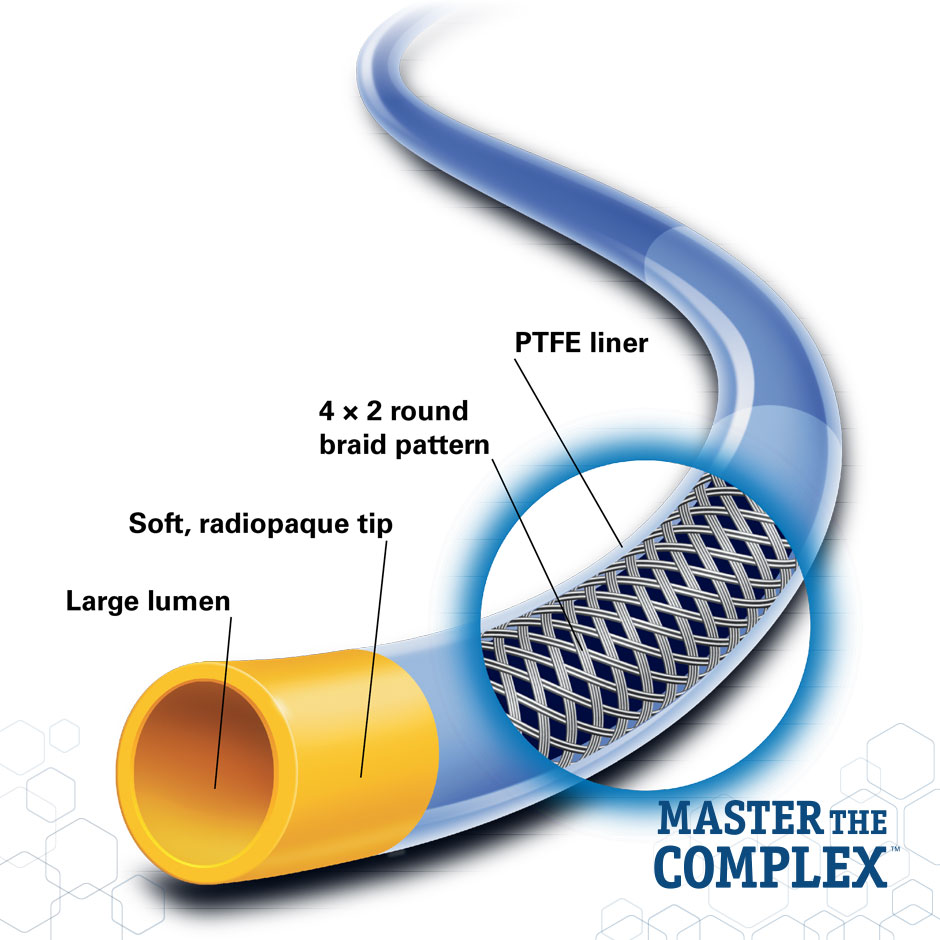
Large Lumen Design
- Offers the device compatibility needed for a multitude of treatment options and treatment scenarios
- Provides improved dye flow for better visualization
Improved Curve Retention and Tip Softness*
- Maintains primary curve shape 40% better than the Launcher Guide Catheter
- 40% softer tip than the Launcher Guide Catheter for improved placement confidence
Design and Performance Dynamics
- Firm catheter shaft for users preferring a passive guide catheter
- Available in 6F lumen size (0.070")
* Bench test data on file. N=22 for Launcher; N=15 for RUNWAY. Bench test results may not necessarily be indicative of clinical performance.
Curve Retention: Testing measures the ability of the catheter to effectively retain its curve shape while placed in water at body temperature (37° C). Smaller changes in the curve angles may increase the catheter’s ability to direct the therapy device to the intended target site. Tip Softness: Testing measures the force absorbed by the catheter’s tip when compressed or deflected at 0.02". Lower force indicates a softer tip.
Reimbursement
The C-code used for this product is C1887: Catheter, guiding (may include infusion/perfusion capability). C-codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific is not responsible for the correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.