NC EMERGE™
PTCA Dilatation Catheter
The Future Built on a Legacy
For decades, we have worked together to define the future. By bringing technology and performance together, we continue our commitment to evolving balloon catheter technology.
Product Details
NC EMERGE is Boston Scientific's Most Advanced PTCA Catheter
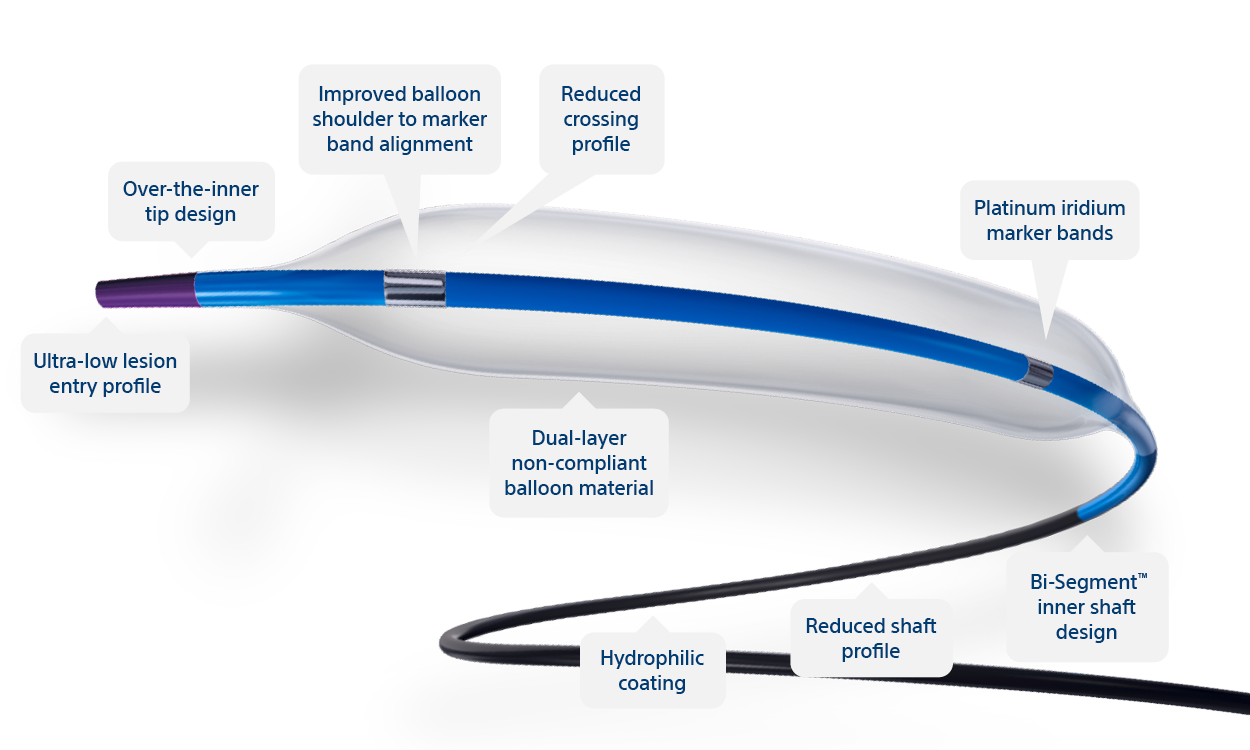
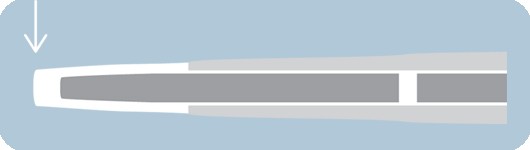
Ultra-low lesion entry point
- 0.017" lesion entry profile
- Improves overall flexibility and performance in tortuous anatomy
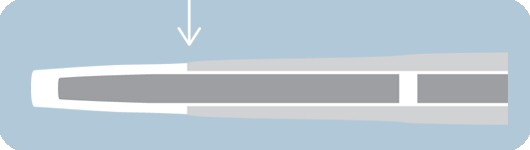
Over-the-inner tip design
- Outer tip material rides over the inner shaft
- Designed to improve overall flexibility and tip performance
- Short tip designed to lessen tip catch occurrence and offer greater control
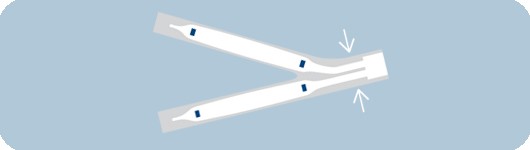
Reduced shaft profile
- Designed for exceptional simultaneous use performance
- Allows for use of two MonorailTM catheters in a 6 F
guide catheter and two Over-the-Wire catheters in an 8 F guide catheter*
Hydrophilic coating
- Reduced frictional force on the catheter shaft
Reduced crossing profile†
- 0.031" (0.787 mm) crossing profile
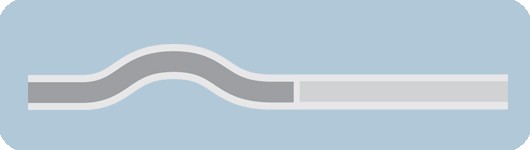
Bi-Segment™ inner shaft design
- Designed for maximum deliverability
- Both stiff and flexible segments to enhance pushability and trackability
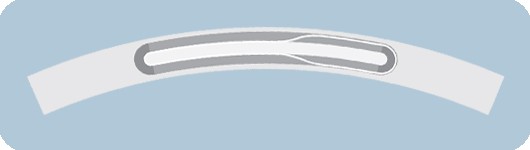
Slope™ outer shaft
- One piece outer shaft provides a seamless transition
- Designed to optimize pushability
- Slope outer shaft on OTW devices

Non-compliant balloon material
- Designed for less balloon growth and increased rated
burst pressure - Unique blend of balloon materials provides excellent
re-wrap
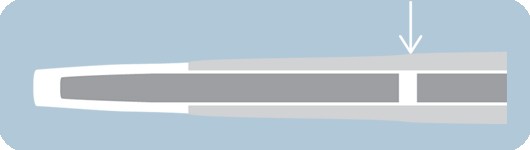
Platinum iridium marker bands
- Provides optimal radiopacity and excellent visibility
Ordering Information
| Monorail | Balloon Length | |||||
| Balloon Diameter (mm) | 6 mm | 8 mm | 12 mm | 15 mm | 20 mm | 30 mm |
| 2 | H7493926706200 | H7493926708200 | H7493926712200 | H7493926715200 | H7493926720200 | H7493926730200 |
| 2.25 | H7493926706220 | H7493926708220 | H7493926712220 | H7493926715220 | H7493926720220 | H7493926730220 |
| 2.5 | H7493926706250 | H7493926708250 | H7493926712250 | H7493926715250 | H7493926720250 | H7493926730250 |
| 2.75 | H7493926706270 | H7493926708270 | H7493926712270 | H7493926715270 | H7493926720270 | H7493926730270 |
| 3 | H7493926706300 | H7493926708300 | H7493926712300 | H7493926715300 | H7493926720300 | H7493926730300 |
| 3.25 | H7493926706320 | H7493926708320 | H7493926712320 | H7493926715320 | H7493926720320 | H7493926730320 |
| 3.5 | H7493926706350 | H7493926708350 | H7493926712350 | H7493926715350 | H7493926720350 | H7493926730350 |
| 3.75 | H7493926706370 | H7493926708370 | H7493926712370 | H7493926715370 | H7493926720370 | H7493926730370 |
| 4 | H7493926706400 | H7493926708400 | H7493926712400 | H7493926715400 | H7493926720400 | H7493926730400 |
| 4.5 | H7493926706450 | H7493926708450 | H7493926712450 | H7493926715450 | H7493926720450 | |
| 5 | H7493926706500 | H7493926708500 | H7493926712500 | H7493926715500 | H7493926720500 | |
| 5.5 | H7493926708550 | H7493926712550 | H7493926715550 | H7493926720550 | ||
| 6 | H7493926708600 | H7493926712600 | H7493926715600 | H7493926720600 | ||
| OTW | Balloon Length | |||||
| Balloon Diameter (mm) | 6 mm | 8 mm | 12 mm | 15 mm | 20 mm | 30 mm |
| 2 | H7493926806200 | H7493926808200 | H7493926812200 | H7493926815200 | H7493926820200 | H7493926830200 |
| 2.25 | H7493926806220 | H7493926808220 | H7493926812220 | H7493926815220 | H7493926820220 | H7493926830220 |
| 2.5 | H7493926806250 | H7493926808250 | H7493926812250 | H7493926815250 | H7493926820250 | H7493926830250 |
| 2.75 | H7493926806270 | H7493926808270 | H7493926812270 | H7493926815270 | H7493926820270 | H7493926830270 |
| 3 | H7493926806300 | H7493926808300 | H7493926812300 | H7493926815300 | H7493926820300 | H7493926830300 |
| 3.25 | H7493926806320 | H7493926808320 | H7493926812320 | H7493926815320 | H7493926820320 | H7493926830320 |
| 3.5 | H7493926806350 | H7493926808350 | H7493926812350 | H7493926815350 | H7493926820350 | H7493926830350 |
| 3.75 | H7493926806370 | H7493926808370 | H7493926812370 | H7493926815370 | H7493926820370 | H7493926830370 |
| 4 | H7493926806400 | H7493926808400 | H7493926812400 | H7493926815400 | H7493926820400 | H7493926830400 |
| 4.5 | H7493926806450 | H7493926808450 | H7493926812450 | H7493926815450 | H7493926820450 | |
| 5 | H7493926806500 | H7493926808500 | H7493926812500 | H7493926815500 | H7493926820500 | |
| 5.5 | H7493926808550 | H7493926812550 | H7493926815550 | H7493926820550 | ||
| 6 | H7493926808600 | H7493926812600 | H7493926815600 | H7493926820600 | ||
Reimbursement
The C-Code used for NC EMERGE PTCA Dilatation Catheter is C1725 Catheter, Transluminal, Angioplasty, Non-Laser (may include guidance, infusion/perfusion capability). C-Codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific Corporation is not responsible for correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.


