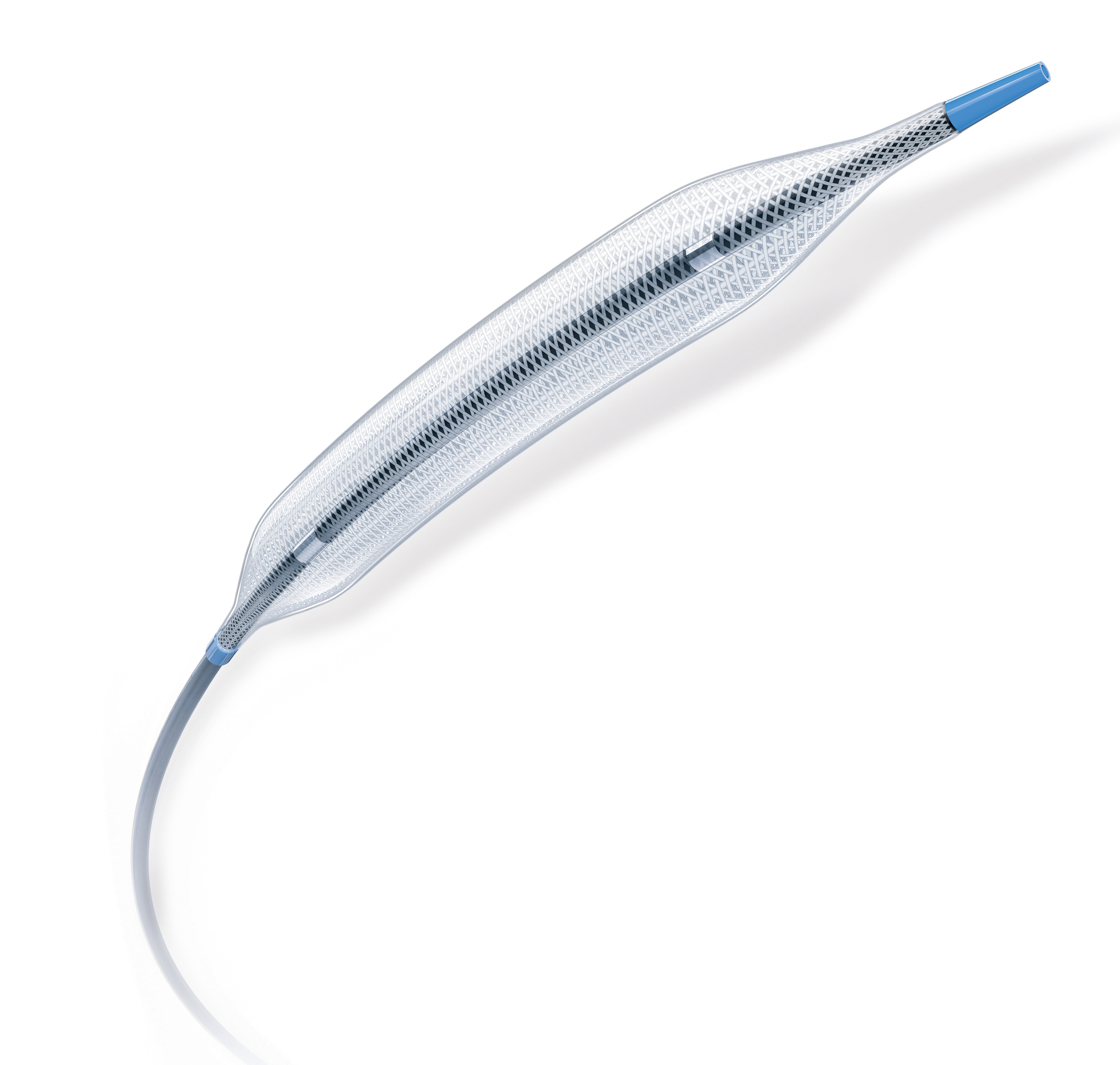
Athletis™
Ultra-High-Pressure Balloon
With pressures up to 40 ATM and a class-leading low lesion entry profile, Athletis Ultra-High-Pressure Balloon offers optimized deliverability to reach and treat highly calcified, long, stenotic, or resistant fibrotic lesions.
Product Details
Ultra-High Pressure:
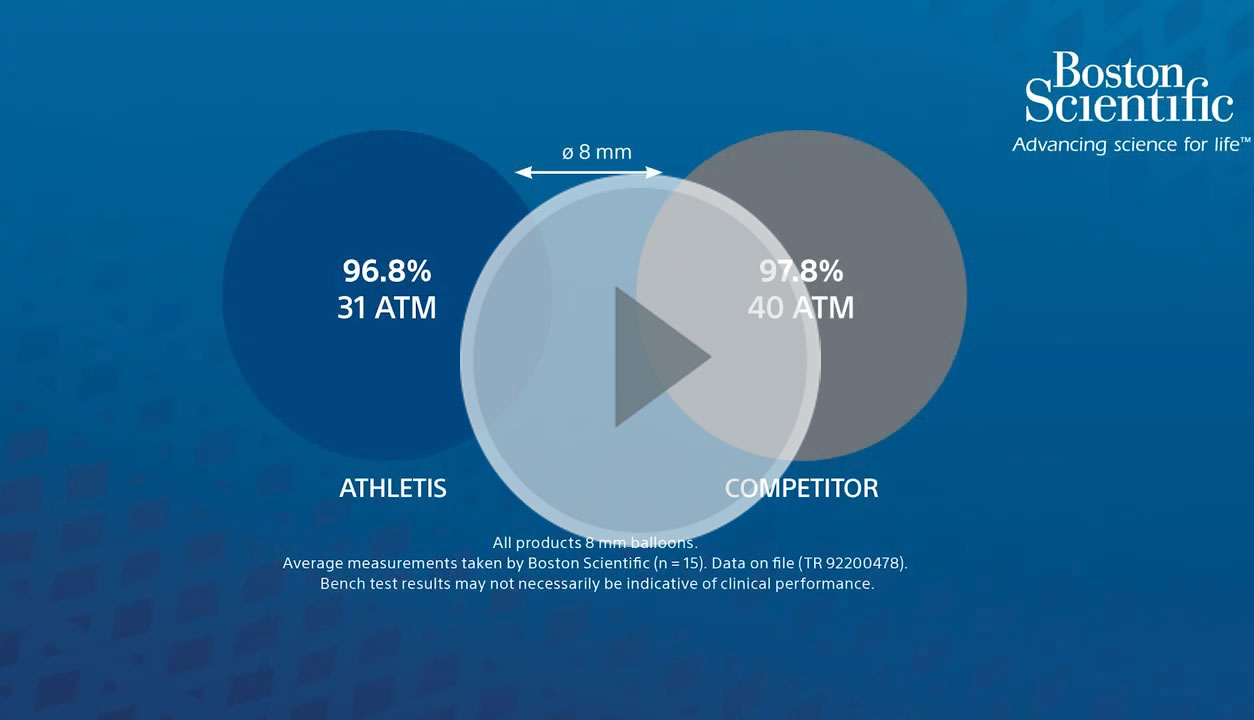
Athletis PTA Balloon’s proprietary braided balloon design enables optimized deliverability and ultra-high pressures up to 40 ATM. The unique, non-compliant balloon design enables Athletis PTA Balloon to open to the stated diameter even in highly resistant lesions.
Ultra-Low Profile:
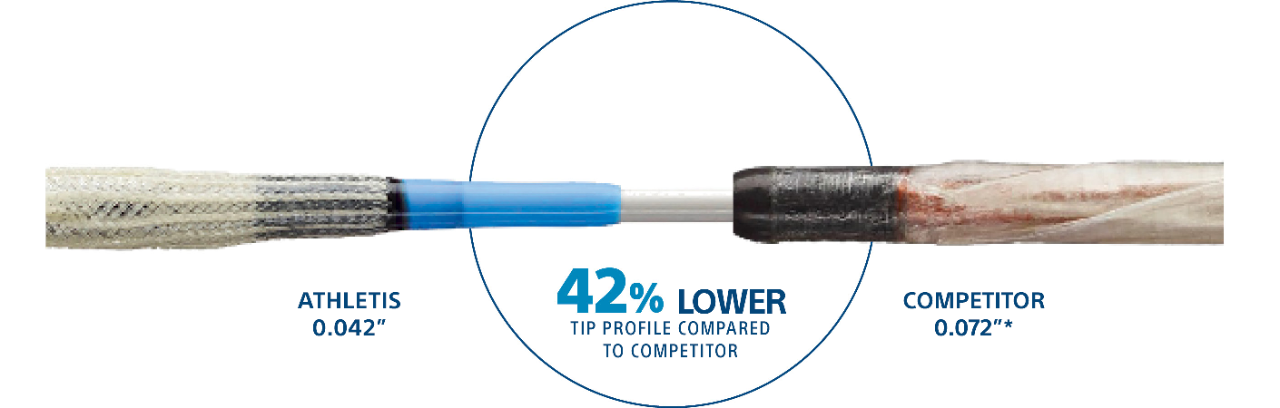
Athletis Ultra-High-Pressure Balloon features a class-leading low lesion entry profile with an 0.042" flexible, tapered tip over an 0.035" guidewire.
Optimized Deliverability:
With an ideal blend of track and a low crossing profile, Athletis PTA Balloon is designed to reach highly calcified, long, stenotic, or resistant fibrotic lesions, including those commonly found in AV access procedures.
Case Study
AV Access Intervention with Ultra-High-Pressure Balloon
Prof. Andrew Holden demonstrates AV Access intervention utilizing ultra-high-pressure balloon angioplasty.
Physician Perspectives
Ordering information
| Balloon Diameter (mm) | Catheter Working Length (cm) | Balloon Length (20 mm) | ||
| 4 | 6F | 50 | UPN GTIN | H74939347040250 08714729973539 |
| 6F | 75 | UPN GTIN | H74939347040270 08714729973546 | |
| 5 | 6F | 50 | UPN GTIN | H74939347050250 08714729973638 |
| 6F | 75 | UPN GTIN | H74939347050270 08714729973645 | |
| 6 | 6F | 50 | UPN GTIN | H74939347060250 08714729973751 |
| 6F | 75 | UPN GTIN | H74939347060270 08714729973768 | |
| 6F | 135 | UPN GTIN | H74939347060210 08714729973744 | |
| 7 | 6F | 50 | UPN GTIN | H74939347070250 08714729973874 |
| 6F | 75 | UPN GTIN | H74939347070270 08714729973881 | |
| 6F | 135 | UPN GTIN | H74939347070210 08714729973867 | |
| 8 | 6F | 50 | UPN GTIN | H74939347080250 08714729973997 |
| 6F | 75 | UPN GTIN | H74939347080270 08714729974000 | |
| 6F | 135 | UPN GTIN | H74939347080210 08714729973980 | |
| 9 | 7F | 50 | UPN GTIN | H74939347090250 08714729974123 |
| 7F | 75 | UPN GTIN | H74939347090270 08714729974130 | |
| 10 | 7F | 75 | UPN GTIN | H74939347100270 08714729974192 |
| 12 | 8F | 75 | UPN GTIN | H74939347120270 08714729974246 |
| 8F | 135 | UPN GTIN | H74939347120210 08714729974239 |
| Balloon Diameter (mm) | Catheter Working Length (cm) | Balloon Length (30 mm) | ||
| 8 | 6F | 50 | UPN GTIN | H74939347080350 |
| 6F | 75 | UPN GTIN | H74939347080370 |
| Balloon Diameter (mm) | Catheter Working Length (cm) | Balloon Length (40 mm) | ||
| 4 | 6F | 50 | UPN GTIN | H74939347040450 |
| 6F | 75 | UPN GTIN | H74939347040470 08714729973560 | |
| 5 | 6F | 50 | UPN GTIN | H74939347050450 08714729973669 |
| 6F | 75 | UPN GTIN | H74939347050470 08714729973676 | |
| 6F | 135 | UPN GTIN | H74939347050410 08714729973652 | |
| 6 | 6F | 50 | UPN GTIN | H74939347060450 08714729973782 |
| 6F | 75 | UPN GTIN | H74939347060470 08714729973799 | |
| 6F | 135 | UPN GTIN | H74939347060410 08714729973775 | |
| 7 | 6F | 50 | UPN GTIN | H74939347070450 08714729973904 |
| 6F | 75 | UPN GTIN | H74939347070470 08714729973911 | |
| 6F | 135 | UPN GTIN | H74939347070410 08714729973898 | |
| 8 | 6F | 50 | UPN GTIN | H74939347080450 08714729974048 |
| 6F | 75 | UPN GTIN | H74939347080470 08714729974055 | |
| 6F | 135 | UPN GTIN | H74939347080410 08714729974031 | |
| 9 | 7F | 50 | UPN GTIN | H74939347090450 08714729974154 |
| 7F | 75 | UPN GTIN | H74939347090470 08714729974161 | |
| 7F | 135 | UPN GTIN | H74939347090410 08714729974147 | |
| 10 | 7F | 75 | UPN GTIN | H74939347100470 08714729974215 |
| 7F | 135 | UPN GTIN | H74939347100410 08714729974208 | |
| 12 | 8F | 75 | UPN GTIN | H74939347120470 08714729974260 |
| 8F | 135 | UPN GTIN | H74939347120410 08714729974253 |
| Balloon Diameter (mm) | Catheter Working Length (cm) | Balloon Length (60 mm) | ||
| 4 | 6F | 50 | UPN GTIN | H74939347040650 08714729973577 |
| 6F | 75 | UPN GTIN | H74939347040670 08714729973584 | |
| 5 | 6F | 50 | UPN GTIN | H74939347050650 08714729973683 |
| 6F | 75 | UPN GTIN | H74939347050670 08714729973690 | |
| 6 | 6F | 50 | UPN GTIN | H74939347060650 08714729973805 |
| 6F | 75 | UPN GTIN | H74939347060670 08714729973812 | |
| 7 | 6F | 50 | UPN GTIN | H74939347070650 08714729973928 |
| 6F | 75 | UPN GTIN | H74939347070670 08714729973935 | |
| 8 | 6F | 50 | UPN GTIN | H74939347080650 08714729974062 |
| 6F | 75 | UPN GTIN | H74939347080670 08714729974079 | |
| 12 | 8F | 75 | UPN GTIN | H74939347120670 08714729974284 |
| 8F | 135 | UPN GTIN | H74939347120610 08714729974277 |
| Balloon Diameter (mm) | Catheter Working Length (cm) | Balloon Length (80 mm) | ||
| 4 | 6F | 50 | UPN GTIN | H74939347040850 08714729973591 |
| 6F | 75 | UPN GTIN | H74939347040870 08714729973607 | |
| 5 | 6F | 50 | UPN GTIN | H74939347050850 08714729973706 |
| 6F | 75 | UPN GTIN | H74939347050870 08714729973713 | |
| 6 | 6F | 50 | UPN GTIN | H74939347060850 08714729973829 |
| 6F | 75 | UPN GTIN | H74939347060870 08714729973836 | |
| 7 | 6F | 50 | UPN GTIN | H74939347070850 08714729973942 |
| 6F | 75 | UPN GTIN | H74939347070870 08714729973959 | |
| 8 | 6F | 50 | UPN GTIN | H74939347080850 08714729974086 |
| 6F | 75 | UPN GTIN | H74939347080870 08714729974093 | |
| 9 | 7F | 50 | UPN GTIN | H74939347090850 08714729974178 |
| 7F | 75 | UPN GTIN | H74939347090870 08714729974185 | |
| 10 | 7F | 75 | UPN GTIN | H74939347100870 08714729974222 |
| Balloon Diameter (mm) | Catheter Working Length (cm) | Balloon Length (100 mm) | ||
| 4 | 6F | 50 | UPN GTIN | H74939347041050 08714729973614 |
| 6F | 75 | UPN GTIN | H74939347041070 08714729973621 | |
| 5 | 6F | 50 | UPN GTIN | H74939347051050 08714729973720 |
| 6F | 75 | UPN GTIN | H74939347051070 08714729973737 | |
| 6 | 6F | 50 | UPN GTIN | H74939347061050 08714729973843 |
| 6F | 75 | UPN GTIN | H74939347061070 08714729973850 | |
| 7 | 6F | 50 | UPN GTIN | H74939347071050 08714729973966 |
| 6F | 75 | UPN GTIN | H74939347071070 08714729973973 | |
| 8 | 6F | 50 | UPN GTIN | H74939347081050 08714729974109 |
| 6F | 75 | UPN GTIN | H74939347081070 08714729974116 |
Inflation Device Ordering Information
Encore 40 is the recommended inflation device for Athletis. To order, use UPN M001394472540.
Reimbursement
The C-code used for this product is C1725, Catheter, transluminal angioplasty, non-laser. C-codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific is not responsible for the correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.
Resources
PDF (2.4 MB)
Peripheral Interventions Product Catalog
PDF (4.3 MB)