
Cardiac resynchronization therapy (CRT) devices help your heart beat more efficiently and monitor your condition so your doctor can provide the right treatment for you. Find out more about how your device works below.
About heart failure
If you have heart failure, your heart doesn’t pump as well as it should, so your blood doesn’t circulate as well as it should to supply your body with the oxygen and nutrients it needs to thrive.
In a healthy heart, both ventricles (lower part of the heart) pump or beat at exactly the same time in a coordinated way. It’s like making a fist—all of the fingers squeeze in unison. But for many people with heart failure, the ventricles do not pump at the same time. It’s like making a fist just one finger at a time.
What is a CRT device?
A CRT system consists of two components—the pulse generator, or device, and thin, insulated wires called leads. A CRT device delivers tiny amounts of electrical energy to the heart through these leads. This helps restore the normal timing of the heartbeats, causing both ventricles to pump together more efficiently like a fist closing normally again.
How do CRT devices work?
There are two types of CRT devices.
One is a special kind of pacemaker. It’s called a cardiac resynchronization therapy pacemaker (CRT-P) or "biventricular pacemaker." The other is the same device, but it also includes a built-in implantable cardioverter defibrillator (ICD). This type is called a cardiac resynchronization therapy defibrillator (CRT-D).
An implanted CRT-P system
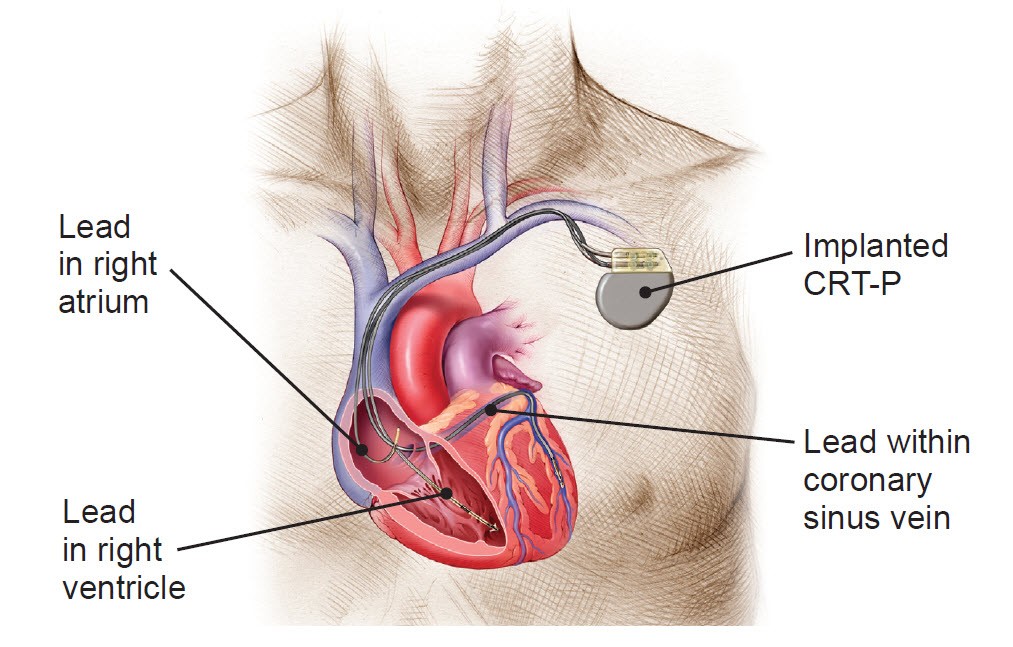
While functioning like a normal pacemaker to treat slow heart rhythms, a CRT-P device also delivers small electrical impulses to the left and right ventricles to help them contract at the same time so your heart pumps more efficiently.
An implanted CRT-D system
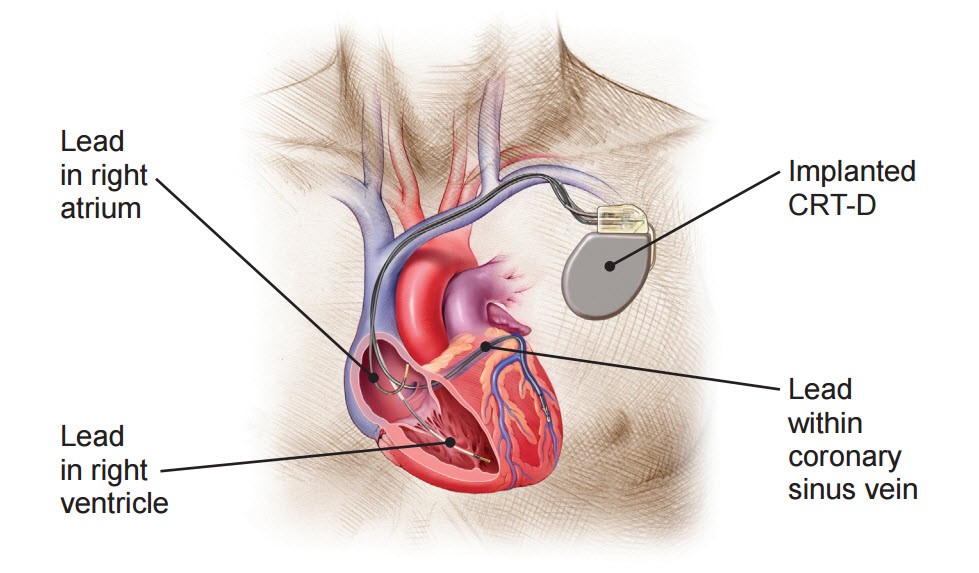
A CRT-D is a special device for heart failure patients who are also at high risk for sudden cardiac death. While functioning like a normal pacemaker to treat slow heart rhythms, a CRT-D device also delivers small electrical impulses to the left and right ventricles to help them contract at the same time. This will help your heart pump more efficiently.
A CRT-D device can also treat dangerously fast heart rhythms (arrhythmias) that can lead to sudden cardiac arrest. If the device senses heartbeats that are dangerously fast, it delivers a shock to the heart. This shock (defibrillation) stops the abnormal rhythm. Without this life-saving therapy, the dangerously rapid rhythm could lead to death in just minutes.
Learn more about your device's battery.
Like any battery, the energy in your implanted device will decrease over time. The battery’s life is affected both by how much energy is programmed to deliver the electrical impulse and how often it is required to pace or deliver therapy to your heart. Your doctor will program the device to meet your individual needs.
Your CRT device will regularly check its own battery and your doctor will check to see how much energy it has left at each follow-up visit. In addition, your doctor can turn on a feature that makes your device beep when replacement time is near. Call your doctor immediately if your device beeps.
Implant risks
While complications don’t happen very often, it’s important to know that there are risks associated with the implantation of any device or lead. You should talk with your health care team about these risks.
See our patient manuals for detailed safety information.