Boston Scientific accounts are for healthcare professionals only.
Editorial commentary
My main goal as a physician treating prostate cancer patients is to not only treat the cancer itself, but also do so in a way that allows the patient to maintain their quality of life. When researching the best way to do this, I rely heavily on data and the results of ongoing clinical trials that evaluate both.
When I began treating patients with SpaceOAR™ Hydrogel, I was doing so as part of a phase III clinical trial that led to the clearance of SpaceOAR Hydrogel by the U.S. Food and Drug Administration (FDA) in 2015.1 Since then, there have been more than 220,000 SpaceOAR Hydrogel units shipped and that number continues to grow.2 Speaking from my clinical experience, both my patients and I find it reassuring that this is not a new-to-the-market product but has been well-vetted over the years with the data to support it.3–5 There are also a number of ongoing studies focused on SpaceOAR Hydrogel that will likely generate additional supporting data.6,7
About the U.S. pivotal trial
The U.S. pivotal trial for SpaceOAR Hydrogel was specifically designed to assess outcomes following spacer implantation.3 The randomized trial enrolled 222 patients at 20 sites across the United States who were being treated for low and intermediate risk prostate cancer.3 By random selection, for every two patients who received SpaceOAR Hydrogel, one patient was assigned to the control group and did not receive a spacer.3 Notably, the first patient enrolled in this trial was at Peninsula Cancer Center (now Seattle Cancer Care Alliance Peninsula) in Poulsbo, Washington where I work as a radiation oncologist.
Each participating site was able to decide whether to administer general anesthesia (36.4%), local anesthesia (31.4%), or MAC — monitored anesthesia care (26%).3 Most of the patients received prophylactic antibiotics and underwent transperineal fiducial marker placement at the same time that the hydrogel spacer was implanted.3
Patient flow
At the beginning of the study, all participants underwent a CT and MRI simulation. They were then randomized and SpaceOAR Hydrogel was placed in the ’SpaceOAR group’ of patients. All of the patients then underwent a second simulation with a CT and MRI scan before dosimetric plans were made. This flow allowed us to compare the scans of patients who did and did not receive SpaceOAR Hydrogel.
Placement results
The results showed that the average thickness of the gel pad was between 10 and 12 mm after placement and this lasted over 12 weeks.3 As patients moved past the three-month mark, it was observed that the gel did start to break down and be absorbed.3 However, this three-month time period allowed me to deliver the full course of external radiation therapy without any substantial change in the thickness of the gel.
With regards to safety, there were no device-related complications and the placement success rate was 99 percent.3 The majority of practitioners also reported that they found the procedure to be easy or very easy to perform.3
Dosimetric outcomes and physician-reported toxicities
The U.S. pivotal trial concluded that increased perirectal space reduced rectal radiation dose, decreased late rectal toxicity scores, and decreased rates of patients experiencing declines in bowel quality of life compared to the control group who did not receive a rectal spacer.3 At the three-year follow-up analysis, there was a significant reduction in late rectal toxicity severity in the SpaceOAR group when compared to the control arm.4 In fact, the dosimetric results showed that the rectal V50 thru V80 was significantly reduced — with a 79 percent reduction in the V70.4 Additionally, with regards to physician-reported toxicity at three years, there were no grade two or higher toxicities reported in the SpaceOAR group.4 Grade two toxicity means any toxicity that needs some form of therapy to address the symptoms.
There was a transient decrease in patient-reported bowel quality of life in both groups initially, but the majority of patients in the SpaceOAR group returned to baseline.3 There were, however, some patients in the control group who did not return to baseline bowel quality of life.3 Over the long term, bowel QOL consistently favored the spacer group, with the difference at 3 years (5.8 points; P<.05) meeting the threshold for a minimally important difference (MID).4
One of the most important takeaways in this study is that SpaceOAR Hydrogel reduced the rectal radiation dose by about 73 percent.3 That translates into a 75 percent reduction in late rectal toxicity4 and also a significant improvement in bowel quality of life.4
Urinary and sexual quality of life findings
Other interesting findings in this study, were the fact that the urinary quality of life reduction and urinary incontinence rates were significantly lower in the SpaceOAR group compared to the control group at three-year median follow-up.4 In fact, a statistically significant difference was found in urinary QOL favoring the spacer arm (+0.6 points) compared with the control arm (-3.3 points; P=.04).4
Additionally, since erections require blood flow through the penile bulb,8 more radiation to the penile bulb may mean reduced blood flow and reduced erectile function.5 Based on patient-reported sexual QOL, results showed that men in the SpaceOAR group who were potent at baseline were more likely to remain so after treatment.5 And, at three years after radiation therapy, 67 percent of these potent men who received SpaceOAR Hydrogel reported that they had erections sufficient for intercourse.5
Use of SpaceOAR Hydrogel with extracapsular extension
The information provided in this editorial represents the opinion of Dr. Hsi. In Canada, SpaceOAR Hydrogel is only approved to be used in prostate cancer patients without evidence of posterior extracapsular extension.
When it comes to the use of SpaceOAR Hydrogel in patients with extracapsular extension (ECE), I tend to consider these on a case-by-case basis. I first attempt to determine the location of the ECE via a multi-parametric prostate MRI. If it is in the anterior or lateral prostate, it’s my opinion that the use of a spacer is usually not a problem.
Should the location of the ECE not be completely conclusive on MRI, I go into the operating room with a plan to place both the gold markers and the spacer but I let the patient know in advance that I will need to make a “game time decision” in the OR. In the procedure, I will examine the posterior prostate with a transrectal ultrasound which allows me to better determine whether the use of SpaceOAR Hydrogel is appropriate and is a viable option for the patient.
This real-time OR approach is only advised for surgeons with a solid knowledge of and experience working with ultrasound anatomy. That’s because the radiologist may see some blur on the image and call it a capsular abutment with possible early ECE. However, that blur could also be a result of motion during the scan because MRI is not always good at differentiating motion artifact and ECE. That’s why it’s essential to use a high-quality ultrasound in the operating room to determine whether or not the ECE is an issue.
This scenario is another reason why it’s critical to have a good relationship with the radiologist reading the MRI scans and, in this situation, to use a radiologist who is experienced or has training in reading multi-parametric MRIs.
The future of perirectal spacing and radiotherapy for prostate cancer
Awareness and validation of SpaceOAR Hydrogel continues to grow.2 It has been mentioned in more than 225 clinical publications to date with numerous additional studies currently underway.2
Notable studies currently underway include the SABRE Trial6 and the POTEN-C Trial.7 The SABRE trial is the first study to evaluate the SpaceOAR Vue™ Hydrogel, the next-generation SpaceOAR Hydrogel designed to offer enhanced visibility via CT scan.2,6 The trial plans to enroll 500 patients globally and is designed to demonstrate the effectiveness of reducing late gastrointestinal toxicity in subjects undergoing stereotactic body radiotherapy to treat prostate cancer.6 This study is the largest SpaceOAR Hydrogel study since the pivotal trial.
The POTEN-C (Prostate Oncologic Therapy While Ensuring Neurovascular Conservation) Trial is a phase II randomized multi-center trial evaluating whether the reduction of dose to, or sparing of, neurovascular structures during stereotactic ablative body radiotherapy for localized prostate cancer will improve retention of sexual potency, while also retaining excellent oncologic control and other secondary health-related quality of life endpoints.7 This study is designed to further help substantiate benefits to sexual function from the SpaceOAR Hydrogel pivotal trial.7
I believe the future of perirectal spacing is very promising. Overall, in my 10 years of using SpaceOAR Hydrogel and seeing my colleagues use it in thousands of patients, I would highly recommend use of this tried-and-true technology. Clinical studies of SpaceOAR Hydrogel have demonstrated efficacy of perirectal spacing and benefits to rectal, urinary, and sexual quality of life.3–5
Related content
References
- SpaceOAR FDA Decision Summary - DEN140030.pdf (fda.gov).
- Data on file with Boston Scientific.
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–977.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985.
- Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018;8:e7–e15.
- Effectiveness of the SpaceOAR Vue System in Subjects With Prostate Cancer Being Treated With Stereotactic Body Radiotherapy (SABRE), ClinicalTrials.gov Identifier: NCT04905069
- Prostate Oncologic Therapy While Ensuring Neurovascular Conservation (POTEN-C), ClinicalTrials.gov Identifier: NCT03525262
- Panchatsharam PK, Durland J, Zito PM. Physiology, Erection. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513278/
Alex Hsi , M.D., is a Boston Scientific consultant and was compensated for his contribution to this editorial. Statements made represent the opinion and experience of Dr. Hsi. Results from case studies are not necessarily predictive of results in other cases. Results in other cases may vary.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
IMPORTANT INFORMATION: These materials are intended to describe common clinical considerations and procedural steps for the use of referenced technologies but may not be appropriate for every patient or case. Decisions surrounding patient care depend on the physician’s professional judgment in consideration of all available information for the individual case.
Boston Scientific (BSC) does not promote or encourage the use of its devices outside their approved labeling. Case studies are not necessarily representative of clinical outcomes in all cases as individual results may vary.
Results from different clinical investigations are not directly comparable. Information provided for educational purposes only.
For information purposes only. The content of this article/publication is under the sole responsibility of its author/publisher and does not represent the opinion of Boston Scientific.
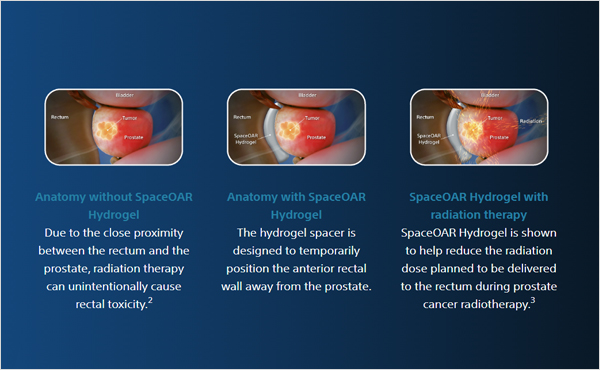
SpaceOAR and SpaceOAR Vue Hydrogels are intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR and SpaceOAR Vue Hydrogels to reduce the radiation dose delivered to the anterior rectum.
SpaceOAR and SpaceOAR Vue Hydrogels contain polyethylene glycol (PEG). SpaceOAR Vue Hydrogel contains iodine.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
As with any medical treatment, there are some risks involved with the use of SpaceOAR and SpaceOAR Vue Hydrogels. Potential complications associated with SpaceOAR and SpaceOAR Vue Hydrogels include, but are not limited to: pain associated with SpaceOAR and SpaceOAR Vue Hydrogels injection, pain or discomfort associated with SpaceOAR and SpaceOAR Vue Hydrogels, local inflammatory reactions, infection (including abscess), urinary retention, urgency, constipation (acute, chronic, or secondary to outlet perforation), rectal tenesmus/muscle spasm, mucosal damage, ulcers, fistula, perforation (including prostate, bladder, urethra, rectum), necrosis, allergic reaction (localized or more severe reaction, such as anaphylaxis), embolism (venous or arterial embolism is possible and may present outside of the pelvis, potentially impacting vital organs or extremities), syncope and bleeding. The occurrence of one or more of these complications may require treatment or surgical intervention. URO-989811-AB.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.