Boston Scientific accounts are for healthcare professionals only.
Summary
- SpaceOAR Vue™ Hydrogel is the next generation of SpaceOAR™ Hydrogel, a polyethylene glycol-based perirectal hydrogel spacer that is designed to position the anterior rectal wall away from the prostate during radiation
- SpaceOAR Vue Hydrogel is not contraindicated for use in patients with known iodine allergy, as the iodine is covalently bound and not in free form
- SpaceOAR Vue Hydrogel is designed to be well-visualized and contoured on CT simulation radiotherapy planning scans since the hydrogel contains covalently bound iodine, similar to intravenous contrast
- MRI may not be required for contouring because SpaceOAR Vue Hydrogel is designed for enhanced visibility on CT, expanding hydrogel spacer use to patients who cannot undergo MRI scans
- SpaceOAR Vue Hydrogel follows the same general placement protocol as SpaceOAR Hydrogel with the major difference that SpaceOAR Vue Hydrogel has increased viscosity and polymerizes more slowly
- SpaceOAR Vue Hydrogel may be easily distinguished from SpaceOAR Hydrogel due to its enhanced visibility on kV cone-beam CT used for daily image-guided radiation therapy
Introduction
Prostate cancer is the most common non-cutaneous cancer diagnosis and second leading cause of cancer death for men in the United States, with over 164,000 new cases and nearly 30,000 deaths estimated for 2020.1 Radiotherapy is a non-invasive treatment alternative to surgery that has comparable clinical outcomes,2,3 while avoiding surgical risks. Radiotherapy is a standard treatment option for patients with prostate cancer.
One drawback of radiotherapy is that healthy tissue surrounding the target area may inadvertently receive radiation, which can lead to side effects.4 When healthy tissue receives radiation, the organ at greatest risk for prostate cancer radiotherapy side effects is the rectum, due to the anatomic proximity of the anterior rectal wall within a few millimeters’ posterior to the prostate.5 Radiotherapy of the prostate is associated with dose-dependent side effects in the GI tract, which are further increased when pelvic lymph nodes are in the treatment field.6–13 Both acute and late GI toxicities correlate with the volume of rectum that is included in the high-dose radiation field of the prostate.4,14 Late rectal toxicity constitutes a spectrum of symptoms that can include increased stool frequency, cramping/tenesmus, fecal incontinence, rectal bleeding, and fistula formation that can significantly reduce a patient’s quality of life following treatment.15,16
Despite improvements in radiotherapy treatment techniques, GI toxicity can be a significant concern for patients undergoing prostate cancer treatment. Late GI toxicity is associated with factors commonly encountered in the prostate cancer patient population, namely advanced age and existing comorbidities.17 Late GI toxicity is associated with the maximal rectal dose and volume of the anterior rectal wall irradiated, which can significantly decrease patient-reported quality of life.4,15,16,18–21 Patient-reported outcomes are valuable metrics for assessing these side effects that often occur long after treatment ends.22,23 Some approaches to increase patient convenience include using hypofractionated and ultra-hypofractionated (i.e., stereotactic body radiation therapy, SBRT) regimens, which are associated with comparable biochemical recurrence-free survival but may still be associated with increased late GI toxicity.24–29 Fortunately, there are techniques that can limit the exposure of healthy GI tissue during radiation therapy for prostate cancer.
One pre-radiation approach to reduce radiation-associated rectal toxicity is to physically increase the distance between the rectum and prostate in a temporary manner.30 The anterior rectal wall is typically within 2–3 mm of the posterior prostate border and is separated from the prostate and bladder by a single multi-layered fibromuscular fascia (i.e., Denonvilliers’). This tissue plane is a potential space that can be artificially expanded using exogenously introduced material such as rapidly polymerizing hydrogel.5 In the authors’ opinion, artificial expansion of the perirectal space prior to starting radiotherapy should:
- Be reproducible
- Be easily and safely performed in the outpatient setting with minimal time investment
- Cause minimal to no patient discomfort while in place
- Remain stable throughout the course of radiotherapy planning and treatment
- Have minimal to no adverse effect on daily setup and treatment
- Biodegrade within a reasonable timeframe following completion of therapy
SpaceOAR™ Hydrogel (OAR = organs at risk) (Boston Scientific, Marlborough, MA) was designed to address these listed characteristics of an ideal spacer. Approved by the Food and Drug Administration (FDA) as well as CE marked, the SpaceOAR System is intended to temporarily displace the anterior rectal wall so as to reduce radiation exposure during prostate cancer radiotherapy treatment. Recently, a second-generation hydrogel spacer, SpaceOAR Vue™ Hydrogel, was developed by Boston Scientific that uses the characteristics of traditional SpaceOAR Hydrogel, while providing a contrast agent designed to be easily tracked by CT imaging. The purpose of this paper is to describe SpaceOAR Hydrogel and to highlight potential key benefits of SpaceOAR Vue Hydrogel for clinicians interested in using a hydrogel spacer for patients undergoing radiation therapy for prostate cancer.
SpaceOAR Hydrogel
SpaceOAR Hydrogel is a polyethylene glycol (PEG) hydrogel injected into the perirectal space prior to radiotherapy that reduces the amount of radiation to the anterior rectal wall through physical displacement of the rectum posteriorly away from the prostate.5 A hydrogel is a material that forms from a chemical reaction of liquid components that crosslink monomeric subunits into polymers. These polymers absorb water and increase to several times the volume of the dissolved monomeric subunits.31 For SpaceOAR Hydrogel, these polymers are comprised of PEG stabilized by crosslinking with trilysine to create a soft but non-deformable hydrogel that is designed to remain stable for approximately 3 months following placement.32
A key point of the SpaceOAR Hydrogel is that polymerization occurs rapidly upon mixing of the solubilized monomers and polymerizing agents. For perirectal spacing, this is accomplished via rapidly mixing the liquid components and injecting them into the perirectal space between Denonvilliers’ fascia and the anterior rectal wall through an aseptic transperineal approach under transrectal ultrasound guidance. Proper placement can be achieved following basic in-office training with a high success rate (> 98%).5 The crosslinks that initially provide the hydrogel with firmness needed to displace the rectum away from the prostate are slowly hydrolyzed in the months after placement and are ultimately removed from the body by the kidneys. SpaceOAR Hydrogel is designed to exhibit complete resorption at 12 months post-placement as assessed by MRI.32
SpaceOAR Hydrogel is designed to successfully displace healthy tissue to decrease the amount of radiation delivered to the rectum. In a pivotal phase 3 trial of SpaceOAR Hydrogel, the distance between the posterior wall of the prostate and anterior rectal wall was increased by a mean of nearly 1.3 cm compared to 0.1 cm in patients with no spacer.5 This distance was slightly less in patients with prostate glands > 80 to 100 cm3 (9.9 mm) and > 100 cm3 (8.8 mm).33
Mariados et al. reported the mean volume of the rectum receiving 70 Gy (V70) had nearly a 10% decrease (12.4% vs. 3.3%, P < 0.001) in patients who had SpaceOAR Hydrogel.5 Patients with larger prostates had a mean rV70 of 2.6% and 2.0% in patients with glands > 80 to 100 cm3 and > 100 cm3, respectively.33 A meta-analysis including over 1000 patients across 7 hydrogel spacer trials demonstrated a mean perirectal separation of 1.1 cm and a 66% reduction in rectal V70.34 Creating space between the prostate and rectum has been shown to significantly decrease the amount of radiation received by rectal tissue.5
Decreasing the amount of radiation received by healthy GI tissue has led to fewer bowel-related side effects. While the benefits of SpaceOAR Hydrogel correlate with a known dosimetric constraint (rectal V70) predictive for rectal toxicity, patients whose treatment plans met established Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) rectal constraints also reported improvement in their symptoms.35 One study demonstrated that patients who had SpaceOAR Hydrogel during radiation therapy underwent significantly fewer endoscopic evaluations and treatment for bowel symptoms by 16% and 11%, respectively.36 Patients who received SpaceOAR Hydrogel and dose-escalated radiotherapy continued to report lower rates of Grade ≥1 (9.2 vs. 2.0%, P = 0.028) and Grade ≥2 (5.7% vs. 0%, P = 0.012) rectal toxicity at 3 years post-treatment compared to patients without a spacer.37 Another study showed a 77% reduction in ≥ Grade 2 long-term rectal side effects.34 A pooled analysis of patient-reported outcomes for bowel-related quality of life showed a continued benefit of reduced bowel urgency, loose stools, and frequency at up to 3 years post treatment.36,38
Using a hydrogel spacer can also decrease radiation reaching other key areas of healthy tissue and improve late-occurring side effects in systems outside of the bowel. On a secondary analysis, SpaceOAR Hydrogel patients exhibited a decreased penile bulb radiation dose and reported improved erectile function, maintenance of potency (66.7% vs. 37.5%, P = 0.046), and higher scores on 7/13 patient-reported sexual health questions (P < 0.05) at 3 years post treatment compared to patients without spacer.39 Since interventions for the late effects of radiotherapy for prostate cancer are not comprehensively studied, and it can be difficult to predict those patients who will experience side effects, use of a hydrogel like SpaceOAR can provide a treatment approach for patients who want to reduce their likelihood of experiencing late side effects.40
SpaceOAR Vue Hydrogel
SpaceOAR Vue Hydrogel received FDA approval in 2019, as well as CE marked in April 2020 and is a modification of standard SpaceOAR Hydrogel, containing approximately 1% iodine by volume covalently bound to the PEG hydrogel.
The addition of iodine to SpaceOAR Vue Hydrogel allows for enhanced viewing on CT planning (average HU of around 300) and daily cone-beam CT scans for image-guided radiation therapy, while the high comparable water content of the hydrogel allows it to retain visibility on T2 MRI like SpaceOAR Hydrogel (Figure 1-B and D). Despite its iodine content, SpaceOAR Vue Hydrogel is not contraindicated in patients with iodine allergy since the iodine is covalently bound to the PEG polymer. SpaceOAR Vue Hydrogel’s design for CT visibility may give physicians another tool if T2 MRI imaging is not an option (Figure 1-C).
With standard SpaceOAR Hydrogel, injection into the perirectal space must be uniform and rapid (within 10 seconds) to reduce polymerization and occlusion of the injection needle. There is little time to correct for hydrogel asymmetry if hydrogel placement is started at an off-axis position. Asymmetric SpaceOAR Hydrogel placement has a small impact on dosimetry, with significant reduction in rectal radiation dose sparing occurring only with lateral hydrogel asymmetry > 2 cm from midline.41 While such deviation has been observed to occur only in a small subset of patients,41 technical considerations such as proper hydrodissection and precise needle placement are beneficial for successful midline placement of the hydrogel. In the authors’ experience, unlike SpaceOAR Hydrogel with its 10-second polymerization rate, SpaceOAR Vue Hydrogel took 20–25 seconds to polymerize after starting the injection, making it more forgiving with regards to suboptimal placement in the perirectal space.
In the authors’ experience, SpaceOAR Vue Hydrogel appears to provide perirectal spacing that is comparable to SpaceOAR Hydrogel. Unpublished data from the first 5 patients (average age = 72.6 ± 7.1 years) who received SpaceOAR Vue Hydrogel prior to external beam radiation (70 Gy/28 fractions) demonstrated an average anterior-posterior displacement of the rectum at mid-gland of 1.44 ± 0.24 cm. For placements 1 cm superior to mid-gland, the average anterior-posterior distance was 1.48 ± 0.40 cm, and for 1 cm inferior to midgland the average anterior-posterior distance was 1.37 ±0.35 cm. The average hydrogel volume contoured on CT simulation scan following injection of 10 mL of SpaceOAR Vue Hydrogel into the perirectal space was 10.50 ± 1.19 cm3. The average HU on CT simulation scans of these 5 patients was 150.92 ± 18.66 HU. Hydrogel placement was symmetric in 5/5 (100%) patients as assessed using a previously published symmetry scoring metric.41 The average radiation dose to 90% of the rectal volume was 223.7 ± 163 cGy. Figure 2 displays the similarity in dose volume histograms (DVH) between SpaceOAR and SpaceOAR Vue Hydrogel. These data show perirectal spacing distance, hydrogel symmetry, and rectal DVH.
Workflow advantages with SpaceOAR Vue Hydrogel vs. SpaceOAR Hydrogel
SpaceOAR Vue Hydrogel affords the opportunity for several changes to the workflow of hydrogel placement, simulation, and daily image-guided radiotherapy (IGRT) including:
- May increase hydrogel availability for practices that do not have access to MRI simulators by avoiding the added cost and inconvenience of MRI since SpaceOAR Vue Hydrogel is designed to be visible on CT
- The radiopacity of SpaceOAR Vue Hydrogel may provide a suitable option to MRI with implanted metallic devices, such as patients with pacemakers or defibrillators, both of which are contraindications to MRI42
- Opportunity to eliminate dependence on MRI simulation and fusion to CT planning scans in order to accurately contour hydrogel within the perirectal space
- May reduce daily treatment localization time by increasing the efficiency of patient repositioning with daily IGRT since SpaceOAR Vue Hydrogel is visible on kV cone-beam CT and may serve as an additional reference
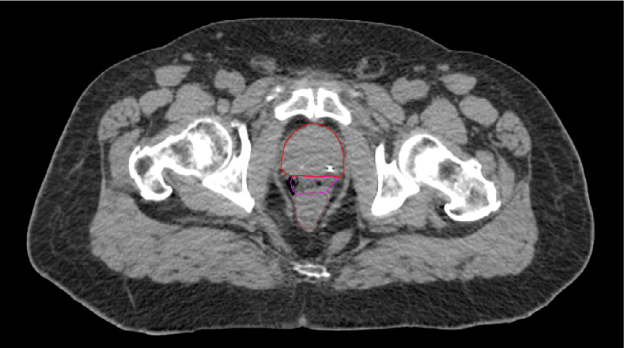
A. SpaceOAR-CT
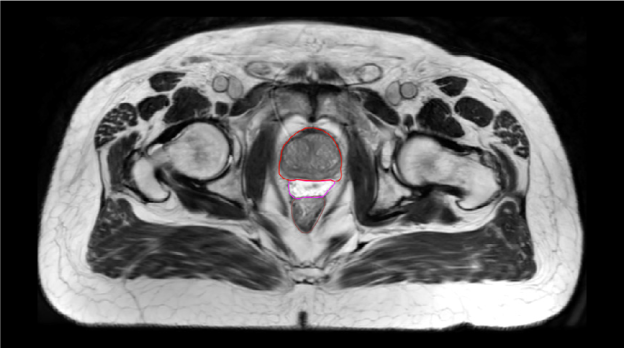
B. SpaceOAR-T2 MRI
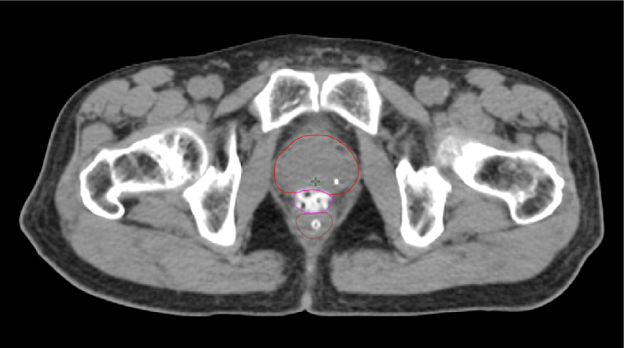
C. SpaceOAR Vue-CT
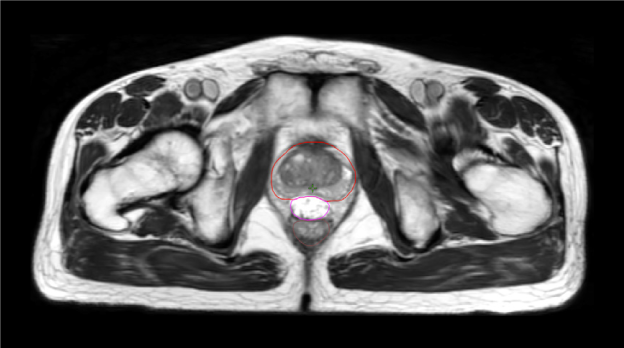
D. SpaceOAR Vue-T2 MRI
SpaceOAR Vue
SpaceOAR
Figure 2. Dose Volume Histograms (DVH) for SpaceOAR Vue Hydrogel and SpaceOAR Hydrogel. Representative DVHs for SpaceOAR Vue Hydrogel (left) and SpaceOAR Hydrogel (right) for the two patients depicted in Figure 1. The DVH lines for rectum (purple) and PTV (prostate and proximal seminal vesicles – cyan) are shown for comparison.
Conclusions
Perirectal hydrogel spacers provide the opportunity for significant rectal sparing from high dose radiation for patients with clinically localized prostate cancer undergoing radiation therapy. SpaceOAR Vue Hydrogel is a new product that incorporates covalently bound iodine into the SpaceOAR PEG-based hydrogel, which has previously been shown to reduce rectal radiation dose and late GI toxicity when placed prior to radiotherapy. Adding the potential for CT visibility to SpaceOAR Vue Hydrogel is anticipated to expand hydrogel accessibility for patients who cannot undergo MRI, eliminate dependence on MRI for hydrogel contouring, assist in daily IGRT, and further improve the convenience of hydrogel placement.
Correspondence to: Dr. Randall Brenneman
Assistant Professor of Radiation Oncology
Department of Radiation Oncology
Washington University School of Medicine, St. Louis, Missouri
Tel.: (314) 362-8567
Fax: (314) 362-7769
E-mail: rbrenneman@wustl.edu
Development of this white paper was financially supported by Boston Scientific.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. https://doi.org/10.3322/caac.21590.
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:479–505. https://doi.org/10.6004/jnccn.2019.0023.
- Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. https://doi.org/10.1056/NEJMoa1606220.
- Olsson CE, Jackson A, Deasy JO, Thor M. A systematic post-QUANTEC review of tolerance doses for late toxicity after prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102:1514–1532. https://doi.org/10.1016/j.ijrobp.2018.08.015.
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–977. https://doi.org/10.1016/j.ijrobp.2015.04.030.
- Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. https://doi.org/10.1016/S0360-3016(02)02829-8.
- Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. https://doi.org/10.1016/j.ijrobp.2007.06.054.
- Hanlon AL, Watkins Bruner D, Peter R, Hanks GE. Quality of life study in prostate cancer patients treated with three-dimensional conformal radiation therapy: Comparing late bowel and bladder quality of life symptoms to that of the normal population. Int J Radiat Oncol Biol Phys. 2001;49:51–59. https://doi.org/10.1016/S0360-3016(00)01365-1.
- Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. J Radiat Oncol Biol Phys. 2008;70:1124–1129. https://doi.org/10.1016/j.ijrobp.2007.11.044.
- Michalski JM, Bae K, Roach M, et al. Long-term toxicity following 3D conformal radiation therapy for prostate cancer from the RTOG 9406 Phase I/II Dose Escalation Study. Int J Radiat Oncol Biol Phys. 2010;76:14–22. https://doi.org/10.1016/j.ijrobp.2009.01.062.
- Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–2332. https://doi.org/10.1200/JCO.2016.67.0448.
- Roach M, Moughan J, Lawton CAF, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1504–1515. https://doi.org/10.1016/S1470-2045(18)30528-X.
- Syndikus I, Morgan RC, Sydes MR, Graham JD; MRC RT01 collaborators. Late gastrointestinal toxicity after dose-escalated conformal radiotherapy for early prostate cancer: results from the UK medical research council RT01 trial (ISRCTN47772397). Int J Radiat Oncol Biol Phys. 2010;77:773–783. https://doi.org/10.1016/j.ijrobp.2009.05.052.
- Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose–volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129. https://doi.org/10.1016/j.ijrobp.2009.03.078.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. https://doi.org/10.1056/NEJMoa074311.
- Nguyen PL, Chen RC, Hoffman KE, et al. Rectal dose–volume histogram parameters are associated with long-term patient-reported gastrointestinal quality of life after conventional and high-dose radiation for prostate cancer: a subgroup analysis of a randomized trial. Int J Radiat Oncol Biol Phys. 2010;78:1081–1085. https://doi.org/10.1016/j.ijrobp.2009.09.015.
- Hamstra DA, Stenmark MH, Ritter T, et al. Age and comorbid illness are associated with late rectal toxicity following dose-escalated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:1246–1253. https://doi.org/10.1016/j.ijrobp.2012.10.042.
- Wang K, Chen RC, Kane BL, et al. Patient and dosimetric predictors of genitourinary and bowel quality of life after prostate SBRT: Secondary analysis of a multi-institutional trial. Int J Radiat Oncol Biol Phys. 2018;102:1430–1437. https://doi.org/10.1016/j.ijrobp.2018.07.191.
- Bruner DW, Pugh SL, Lee WR, et al. Quality of life in patients with low-risk prostate cancer treated with hypofractionated vs conventional radiotherapy: a phase 3 randomized clinical trial. JAMA Oncol. 2019;5:664–670. https://doi.org/10.1001/jamaoncol.2018.6752.
- Alayed Y, Davidson M, Quon H, et al. Dosimetric predictors of toxicity and quality of life following prostate stereotactic ablative radiotherapy. Radiother Oncol. 2020;144:135–140. https://doi.org/10.1016/j.radonc.2019.11.017.
- Murray J, Griffin C, Gulliford S, et al. A randomised assessment of image guided radiotherapy within a phase 3 trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2020;142:62–71. https://doi.org/10.1016/j.radonc.2019.10.017.
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. https://doi.org/10.1056/NEJMoa1606221.
- Heemsbergen WD, Incrocci L, Pos FJ, Heijmen BJM, Witte MG. Local dose effects for late gastrointestinal toxicity after hypofractionated and conventionally fractionated modern radiotherapy for prostate cancer in the HYPRO trial. Front Oncol. 2020;10:469. https://doi.org/10.3389/fonc.2020.00469.
- Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35:1891–1897. https://doi. org/10.1200/JCO.2016.70.4189.
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. https://doi.org/10.1016/S1470-2045(14)70040-3.
- Dearnaley D, Syndikus I, Mossop H, et al; CHHiP Investigators. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. https://doi.org/10.1016/S1470-2045(16)30102-4.
- Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. https://doi.org/10.1200/JCO.2016.71.7397.
- Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. https://doi.org/10.1016/j.ijrobp.2016.11.026.
- Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36:2943–2949. https://doi.org/10.1200/JCO.2018.77.9868.
- Sanei M, Ghaffari H, Ardekani MA, et al. Effectiveness of rectal displacement devices during prostate external-beam radiation therapy: A review. J Cancer Res Ther. 2021;7(2):303–310. DOI: 10.4103/jcrt.JCRT_841_19.
- Data on file with Boston Scientific. Windchill document 92351722TR0031.
- Data on file with Boston Scientific. SpaceOAR BCAR – Formulation and biocompatibility document 92348179.
- Fagundes M, Rodrigues MA, Olszewski S, et al. Expanding the utilization of rectal spacer hydrogel for larger prostate glands (>80 cc): feasibility and dosimetric outcomes. Adv Radiat Oncol. 2021;6:100651. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8233470/.
- Miller LE, Efstathiou JA, Bhattacharyya SK, Payne JA, Woodward E, Pinkawa M. Association of the placement of a perirectal hydrogel spacer with the clinical outcomes of men receiving radiotherapy for prostate cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e208221. https://doi.org/10.1001/jamanetworkopen.2020.8221.
- Quinn TJ, Daignault-Newton S, Bosch W, et al. Who benefits from a prostate rectal spacer? Secondary analysis of a Phase III trial. Pract Radiat Oncol. 2020;10:186–194. https://doi.org/10.1016/j.prro.2019.12.011.
- Pinkawa M, Berneking V, König L, Frank D, Bretgeld M, Eble MJ. Hydrogel injection reduces rectal toxicity after radiotherapy for localized prostate cancer. Strahlenther Onkol. 2017;193:22–28. https://doi.org/10.1007/s00066-016-1040-6.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. https://doi.org/10.1016/j.ijrobp.2016.12.024.
- Seymour ZA, Hamstra DA, Daignault-Newton S, et al. Long-term follow-up after radiotherapy for prostate cancer with and without rectal hydrogel spacer: a pooled prospective evaluation of bowel associated quality of life. BJU Int. 2020;126:367–372. https://doi.org/10.1111/bju.15097.
- Hamstra DA, Mariados N, Sylvester J, et al. Sexual quality of life following prostate intensity modulated radiation therapy (IMRT) with a rectal/prostate spacer: Secondary analysis of a phase 3 trial. Pract Radiat Oncol. 2018;8:e7–e15. https://doi.org/10.1016/j.prro.2017.07.008.
- Porouhan P, Farshchian N, Dayani M. Management of radiation-induced proctitis. J Family Med Prim Care. 2019;8:2173–2178.
- Fischer-Valuck BW, Chundury A, Gay H, et al. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol. 2017;7:195–202. https://doi.org/10.1016/j.prro.2016.10.004.
- ACR Manual on MR Safety. American College of Radiology website. https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf. Published April 2020. Accessed 07/08/2021.
Case studies are not necessarily representative of clinical outcomes in all cases as individual results may vary.
SpaceOAR and SpaceOAR Vue Hydrogels are intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR and SpaceOAR Vue Hydrogels to reduce the radiation dose delivered to the anterior rectum.
SpaceOAR and SpaceOAR Vue Hydrogels contain polyethylene glycol (PEG). SpaceOAR Vue Hydrogel contains iodine. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events.
As with any medical treatment, there are some risks involved with the use of SpaceOAR and SpaceOAR Vue Hydrogels. Potential complications associated with SpaceOAR and SpaceOAR Vue Hydrogels include, but are not limited to: pain associated with SpaceOAR and SpaceOAR Vue Hydrogels injection, pain or discomfort associated with SpaceOAR and SpaceOAR Vue Hydrogels, local inflammatory reactions, infection (including abscess), urinary retention, urgency, constipation (acute, chronic, or secondary to outlet obstruction), rectal tenesmus/muscle spasm, mucosal damage, ulcers, fistula, perforation (including prostate, bladder, urethra, rectum), necrosis, allergic reaction (localized or more severe reaction, such as anaphylaxis), embolism (venous or arterial embolism is possible and may present outside of the pelvis, potentially impacting vital organs or extremities), syncope and bleeding. The occurrence of one or more of these complications may require treatment or surgical intervention.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.