AUSCO Clinical Trial
The Artificial Urinary Clinical Outcomes Clinical Trial is studying the AMS 800™ Artificial Urinary Sphincter (AUS) and its impact on urinary incontinence in men.
The AUSCO trial is currently enrolling patients who plan to receive the AMS 800 in the United States and Australia.
Why AMS 800 AUS?

Find a Trial Site
For more information, or to find out if you're an appropriate candidate for a clinical trial, select the link below and fill out the find a study form.
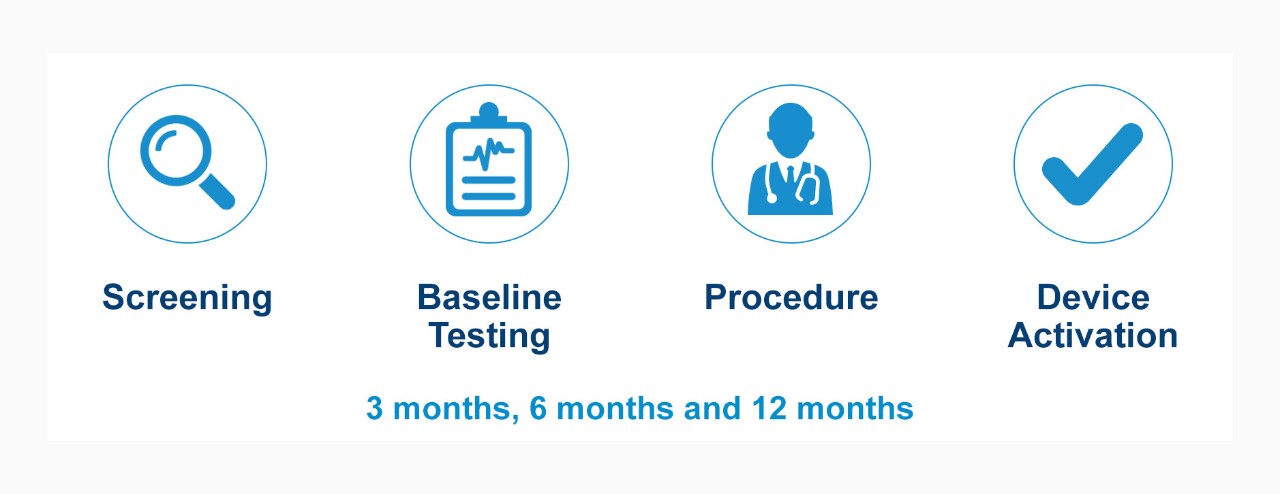
Trial Design and Primary Endpoints
Single-arm, prospective, multi-center study with 12-month follow-up.
Primary Endpoint – Treatment success defined as ≥ 50% reduction in 24-hour pad weight test from baseline at 12 months post device activation.

Trial Timeline (patient timeline)
Who is an AUSCO Patient?
- Primary SUI, De Novo cases, who have undergone either a radical prostatectomy, transurethral resection of the prostate or other invasive prostate surgery
- Positive screening 24-hour pad weight test: Greater than or equal to 100-gram
- Experiences at least 3 incontinence episodes per day during baseline diary
- No current UI devices or comorbidities (known urogenital cancer, urethral stricture, etc.)
