Innovation in atrial fibrillation treatment
A growing number of people across the U.S. are developing atrial fibrillation (AFib), the most common arrhythmia1. Catheter ablation for AFib has shown superior effectiveness over antiarrhythmic drugs in reducing AFib recurrences2, however only a small percentage of AFib patients receive this treatment today.
Early diagnosis followed by safe and effective ablation treatments can slow disease progression, reduce AFib recurrences, and improve patients’ quality of life.3
The burden of atrial fibrillation
12.1 M
U.S. patients with AFib by 20304
1 in 7
Strokes caused by AFib5
>450k
Hospitalizations with AFib annually in US6
Full suite of cardiac ablation therapies.
Boston Scientific is committed to advancing the treatment of Atrial Fibrillation.
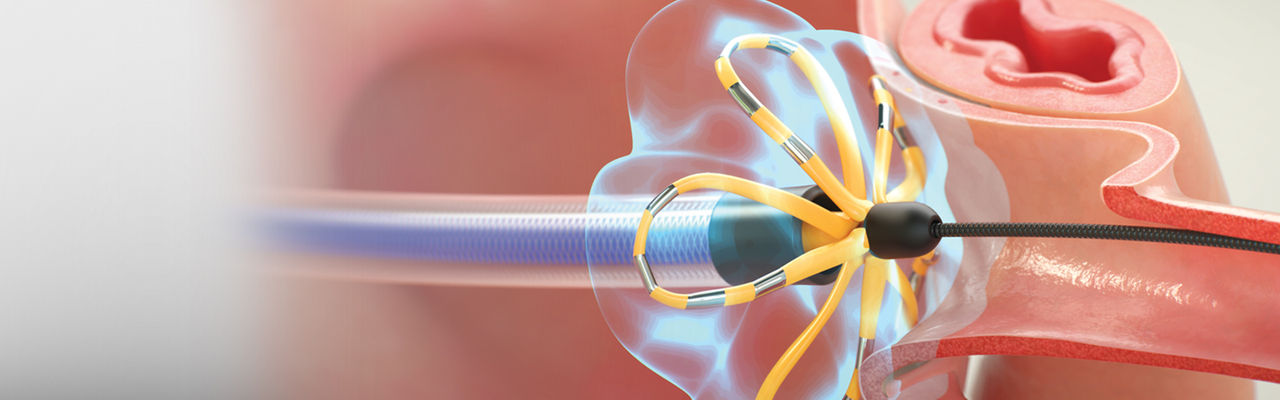
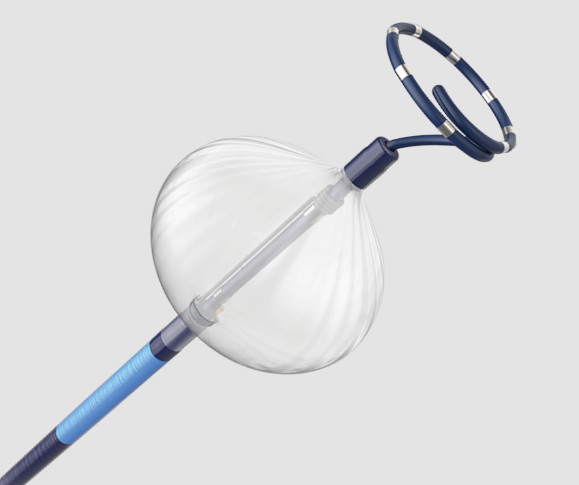
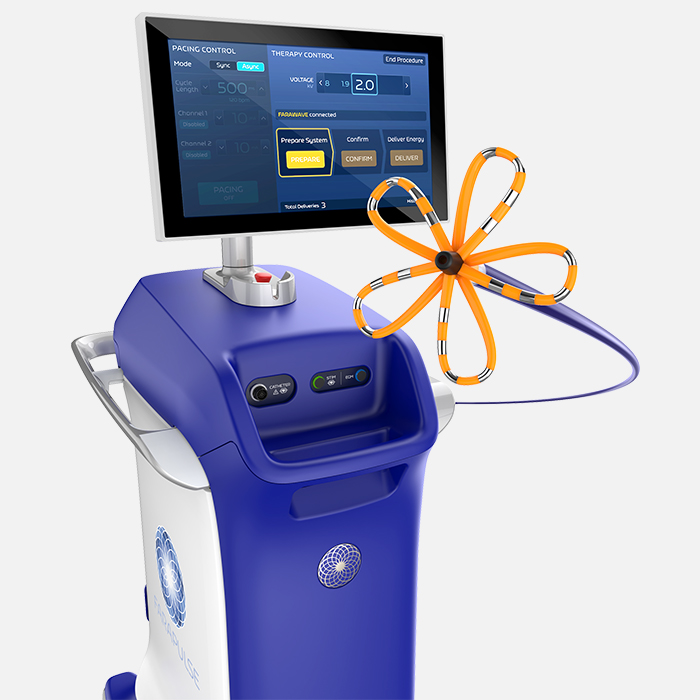
FARAPULSE™ Pulsed Field Ablation System
New! MedAxiom CV Business Paper

Real-World Experience with FARAPULSE™ Pulsed Field Ablation System.
Discover how FARAPULSE™ helped a health system increase their AFib ablation volume by 24% without additional staff or facility requirements.
What is PFA?
Pulsed Field Ablation (PFA) is a non-thermal mechanism of action that has the potential to transform cardiac ablation. Offering several benefits over traditional thermal energy sources, PFA can be optimized to target myocardial tissue while sparing surrounding anatomy.
Why choose FARAPULSE PFA?
FARAPULSE’s proven safety, efficacy, and efficiency are functions of the optimized catheter design, waveform, and dosing strategy.
Unparalleled clinical evidence
- The ADVENT Pivotal Trial, the only randomized controlled trial comparing PFA to cryoablation and radiofrequency ablation, met primary safety and efficacy endpoints while delivering statistically significant improvement in efficiency.7
- MANIFEST-17K is the largest, real-world safety registry of any PFA system.8 In the more than 17k patients studied, zero cases of:
- Esophageal fistula
- Pulmonary vein stenosis
- Phrenic nerve paralysis
Operational efficiencies
- Standardized, simple workflow
- Flexible imaging guidance and compatible with any mapping system
- Reproducible procedure
Economic value for administrators
- 42% average reduction in ablation time7 may increase capacity to add more cases
- Predictable cases improve schedule consistency
Discover how Pulsed Field Ablation with FARAPULSE can positively impact clinical practice and improve efficiencies across your cardiovascular service line (CVSL).

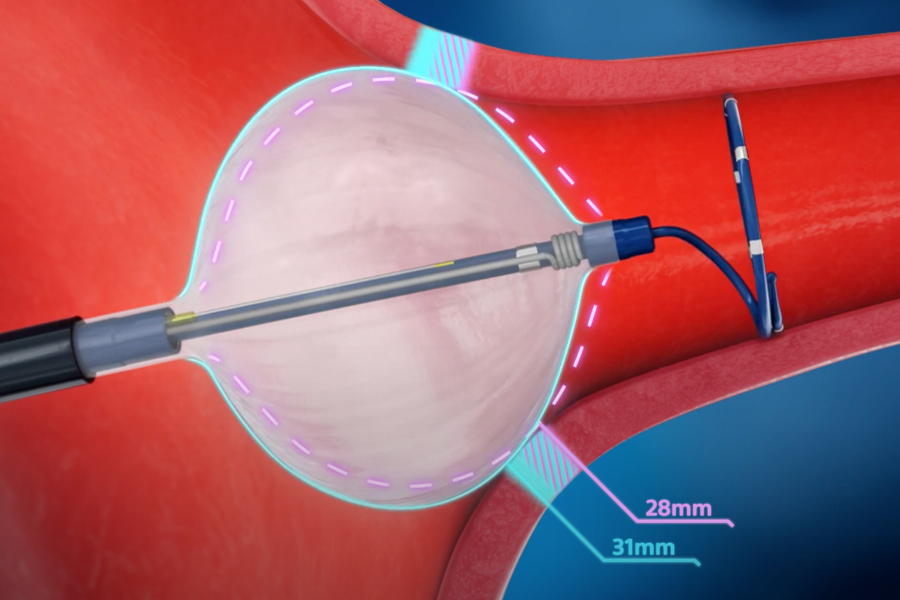
POLARx™ Cryoablation System
The POLARx Cryoablation System redefines what's possible in cardiac cryoablation. Featuring POLARx™ FIT, the only dual-diameter cryoballoon in one catheter, the POLARx System provides:
- Greater occlusion capability
- Greater ablation confidence
- Greater workflow capabilities