Boston Scientific’s portfolio and pipeline, of transcatheter structural heart valve therapies, tools, clinical evidence and training is designed to help you:
- Improve quality outcomes while reducing the risk of transcatheter aortic valve replacement (TAVR)-related stroke and complications
- Improve operational efficiencies through meaningful innovation, training, and education to expand on clinicians’ and lab staff’s expertise
- Advance your structural heart program with creative healthcare solutions and partnerships providing value beyond our market-leading products
Commitment to a patient-first mentality
Protection from stroke
Boston Scientific is the first and only company to offer Protected TAVR™ with SENTINEL™ Cerebral Protection System enabling you to reduce the risk of TAVR-related stroke — a devastating outcome for patients and the healthcare system.
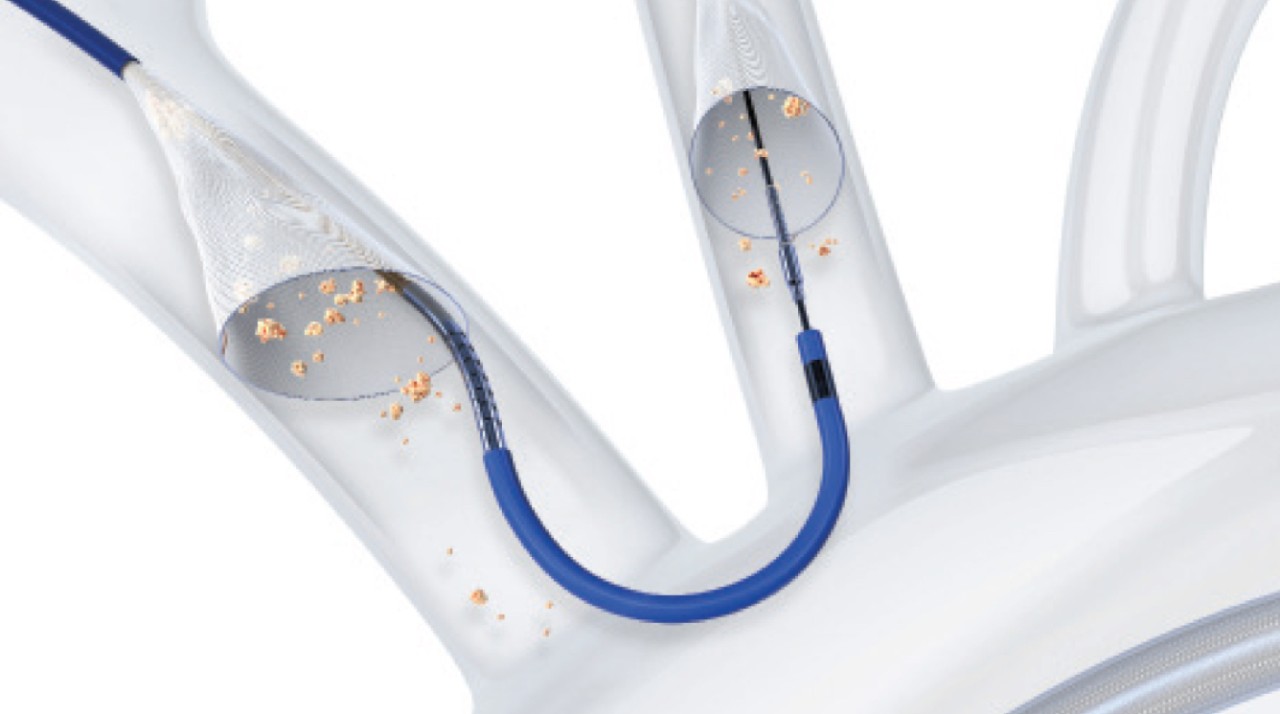
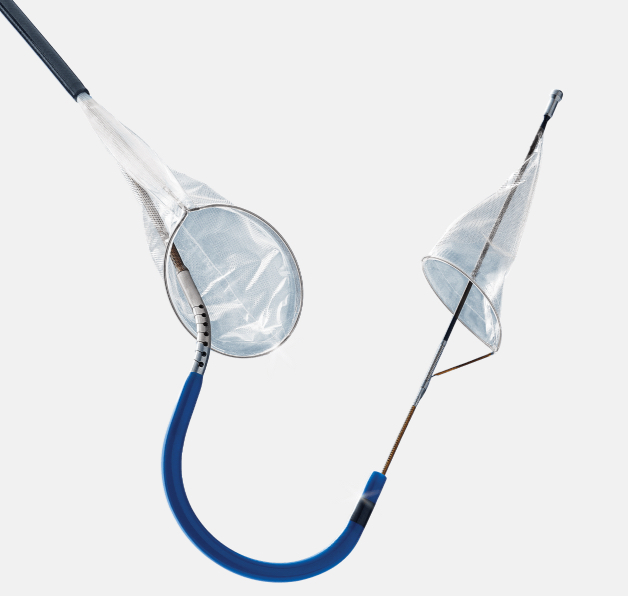
SENTINEL™ Cerebral Protection System
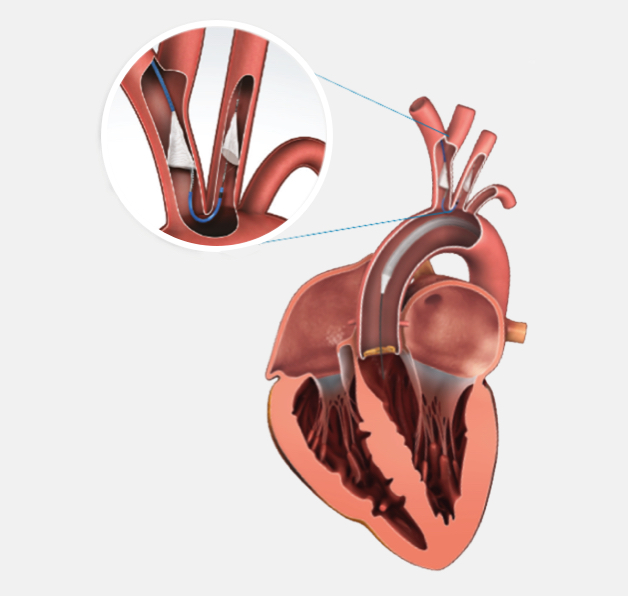
High cost associated with stroke during TAVR
23%
Increase ($12,737) in average TAVR index hospitalization costs5
4.2
Average increase in length of stay (LOS)5
70%
Increase in cumulative 1-year healthcare costs (additional $59,646)5
31%
Higher 30-day readmission rates (20.5% vs 15.6%)6

