ELUVIA™
Drug-Eluting Vascular Stent System
Eluvia provides consistent, durable outcomes in challenging SFA disease and features a polymer design for controlled drug release.
Product Details
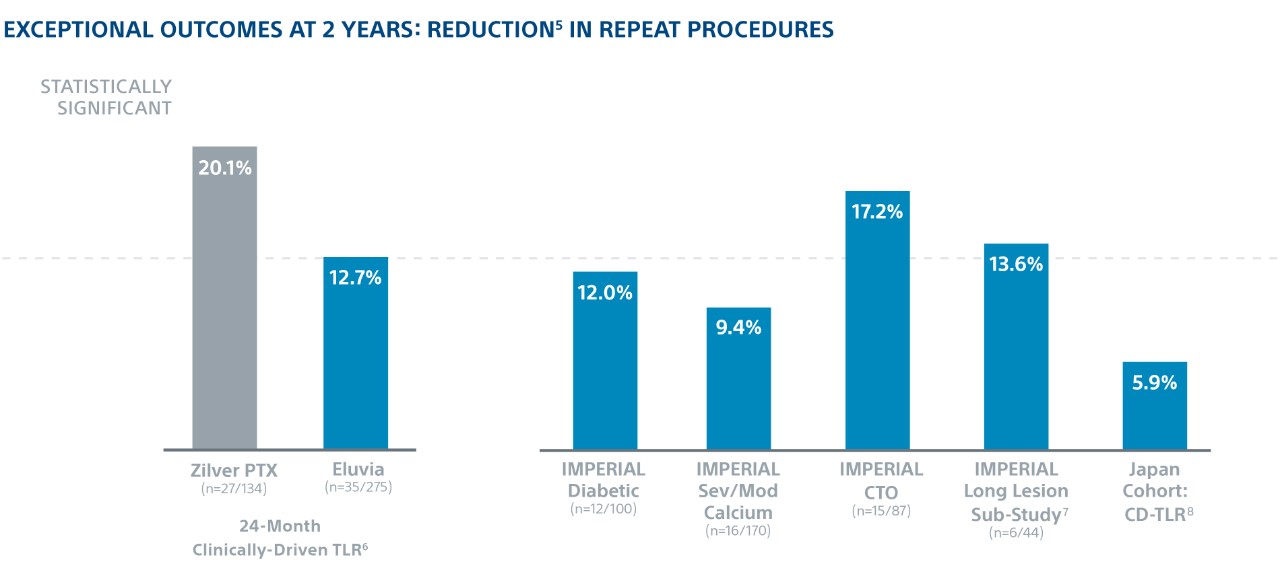
Exceptional Outcomes in Complex Lesions
In the world’s first head-to-head DES SFA trial1, Eluvia demonstrated the highest ever reported 2-year Kaplan-Meier primary Patency of 83%2 and showed low 2-year TLR in patients with long lesions, diabetes, CTOs, and severe/moderate calcium.
As the only device using polymer to elute drug for PAD, Eluvia features the lowest drug dose with the fewest downstream particulates3, leaving no paclitaxel in the bloodstream at 30 minutes post implant.4
Available now in 150mm length. Ask your sales representative about Eluvia 150.
Eluvia™ Drug-Eluting Vascular Stent System UPN and GTIN Codes
| Description | UPN | GTIN |
| Eluvia 6 mm x 40 mm x 130 cm | H74939294600410 | 08714729876571 |
| Eluvia 6 mm x 60 mm x 130 cm | H74939294600610 | 08714729876588 |
| Eluvia 6 mm x 80 mm x 130 cm | H74939294600810 | 08714729876595 |
| Eluvia 6 mm x 100 mm x 130 cm | H74939294601010 | 08714729876601 |
| Eluvia 6 mm x 120 mm x 130 cm | H74939294601210 | 08714729876618 |
| Eluvia 6 mm x 150* mm x 130 cm | H74939294601510 | 08714729876625 |
| Eluvia 7 mm x 40 mm x 130 cm | H74939294700410 | 08714729876694 |
| Eluvia 7 mm x 60 mm x 130 cm | H74939294700610 | 08714729876700 |
| Eluvia 7 mm x 80 mm x 130 cm | H74939294700810 | 08714729876717 |
| Eluvia 7 mm x 100 mm x 130 cm | H74939294701010 | 08714729876724 |
| Eluvia 7 mm x 120 mm x 130 cm | H74939294701210 | 08714729876731 |
| Eluvia 7 mm x 150* mm x 130 cm | H74939294701510 | 08714729876748 |