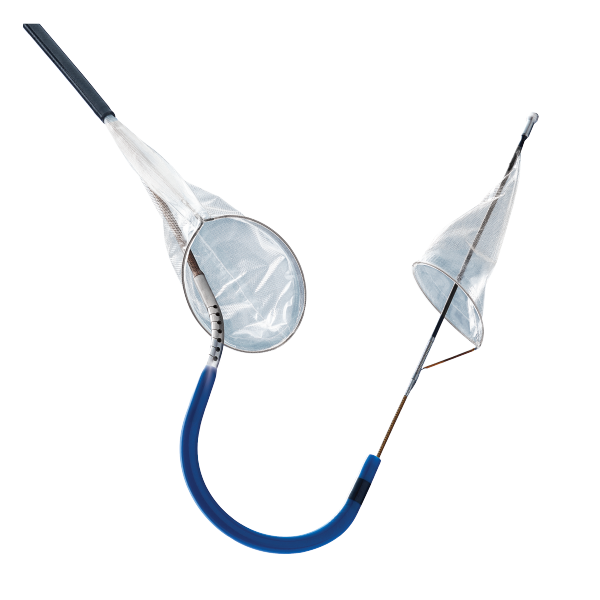
SENTINEL™
Cerebral Protection System
Stroke Happens. PROTECTION Works.
Protected TAVR™ with SENTINEL Cerebral Protection System (CPS) gives you the power to reduce stroke.
Key Resources
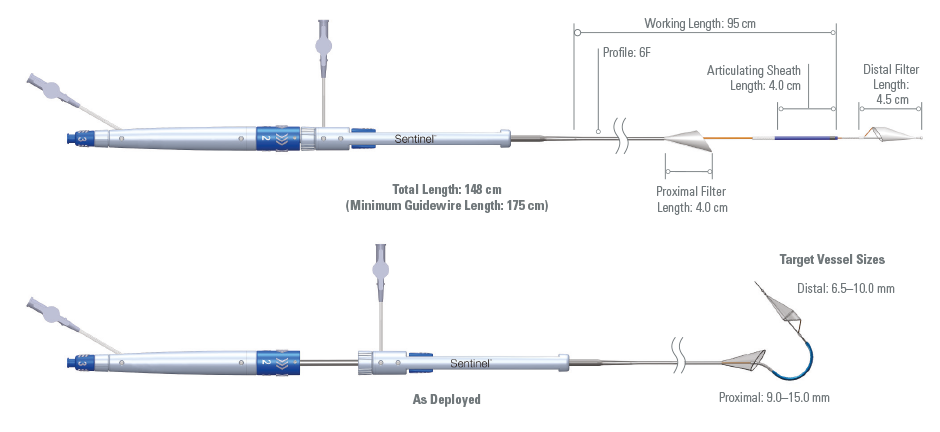
Product Specifications
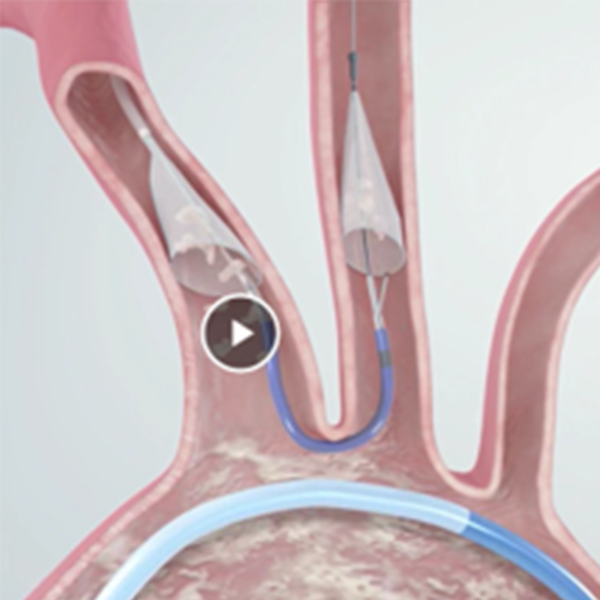
| Indications for Use | The SENTINEL™ Cerebral Protection System is indicated for use as an embolic protection device to capture and remove thrombus/debris while performing TAVR (transcatheter aortic valve replacement) procedures.1 |
| Access Route | Right Radial Access Only |
| Deployment Procedure | Sequential Filter Deployment and Retrieval |
| Guidewire Compatibility | 0.014" (0.36 mm) Diameter Floppy Tip Coronary Guidewire, 175 cm Minimum Length |
| Use | Single Use |
| Sterilization | E-beam Radiation |
| Carton Size | 61.55 in x 1.05 in x 3.55 in |
Ordering Information
Sentinel CPS System Standalone
| Order Number | Ref/Catalog Number | Description | Units |
| 00963229000004 | CMS15-10C-US | SENTINEL Cerebral Protection System | 1 |
Reimbursement
HCPCS Code -C1889 (Implantable/insertable device, not otherwise classified) may be used when appropriate.
ICD-10-PCS Procedure Code X2A5312 (Cerebral Embolic Filtration, Dual filter in Innominate Artery and Left Common Carotid Artery, Percutaneous Approach, New Technology Group 2) may be used to describe TAVR and the use of SENTINEL Cerebral Protection System procedures.
Comprehensive guide providing an overview of the coding, coverage and payment landscape for the SENTINEL™ Cerebral Protection System
For Reimbursement support, email IC.Reimbursement@bsci.com or reach out to your BSC therapy representative