EMBLEM™ MRI S-ICD System
Subcutaneous Implantable Defibrillator
APPRAISE ATP trial: ATP plus shock vs. shock-only
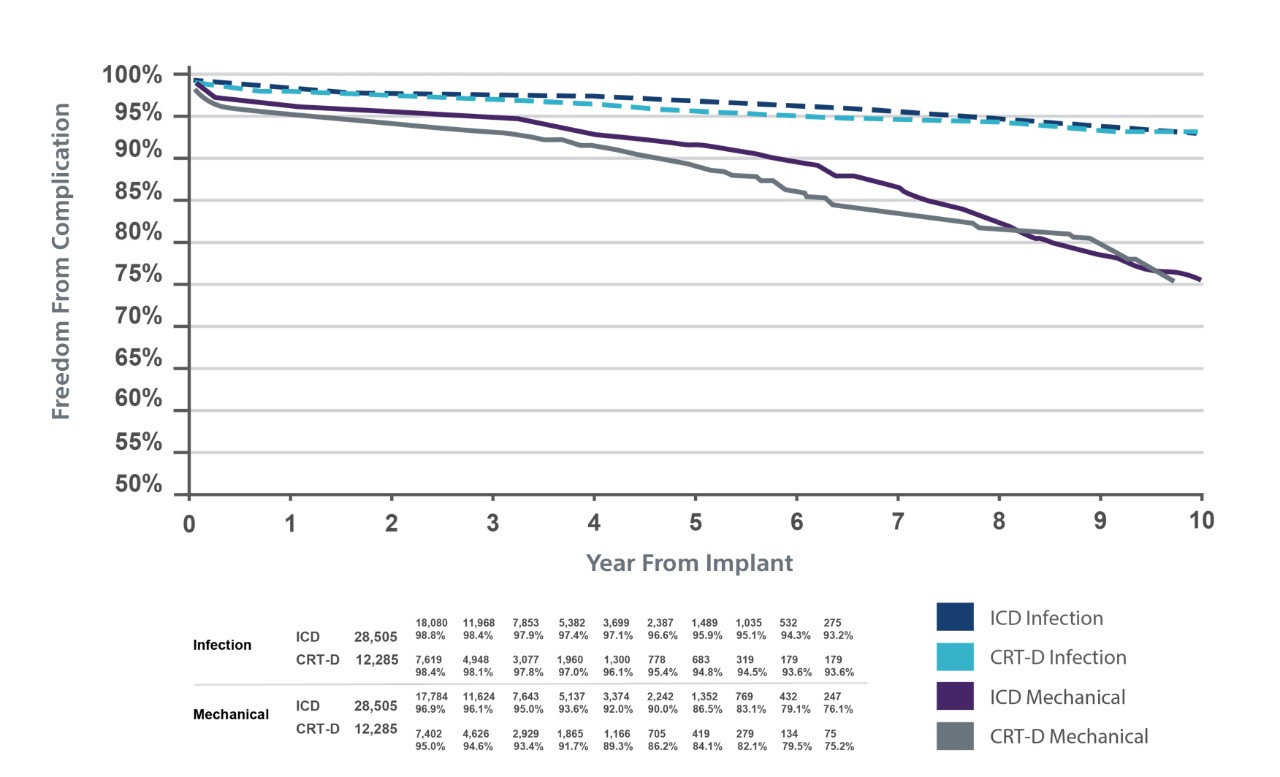
ATLAS Trial: Lead-related complication rates
PRAETORIAN: S-ICD vs. TV-ICD
First results of the randomized PRAETORIAN-DFT trial
Prospective validation of the PRAETORIAN score for prediction of defibrillation test success after subcutaneous ICD implant
The findings from this first analysis demonstrate that a low (<90) PRAETORIAN score is 99% predictive for a successful DFT and a high (≥90) PRAETORIAN score is the strongest predictor for DFT-failure (odds ratio of 34 ).1
While the study investigators concluded their presentation by stating that the PRAETORIAN score could be used as an alternative for DFT in cases when DFT-testing is not possible or desirable, omitting DFT’s in S-ICD implants cannot be advised until the full PRAETORIAN-DFT results are available.
Analysis of the UNTOUCHED study
Hypothesis
The incidence of inappropriate shocks in primary prevention, LVEF ≤ 35% patients will be non-inferior to the rate in transvenous ICD patients with similar programming observed in MADIT-RIT Arms B and C.
Study Design
- Follow-up for 18 months
- Device programming with a conditional zone of 200 bpm and a shock zone of 250 bpm
- Primary endpoint of inappropriate shock-free rate at 18 months
- Secondary endpoints of all cause shock-free rate at 18 months and system and procedure complications at 30 days
Key Takeaways
1. In the UNTOUCHED study a subsequent sub-analysis demonstrated that the inappropriate shock rate with SMART Pass On was 1.8% at 1 year for EMBLEM™ S-ICDs. This is the lowest reported inappropriate shock rate for S-ICD, despite a cohort with more left ventricular dysfunction and heart failure.2,3
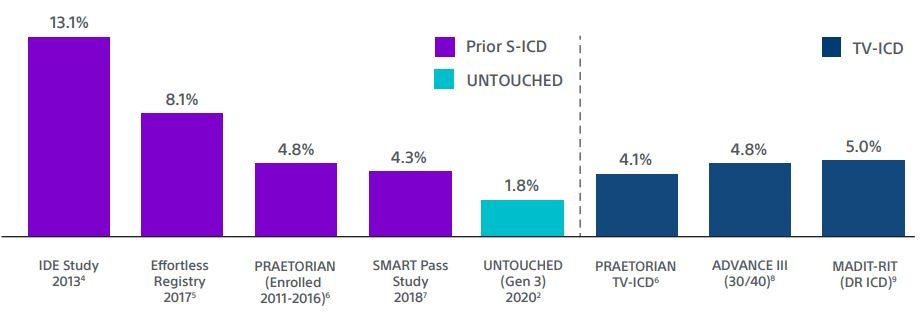
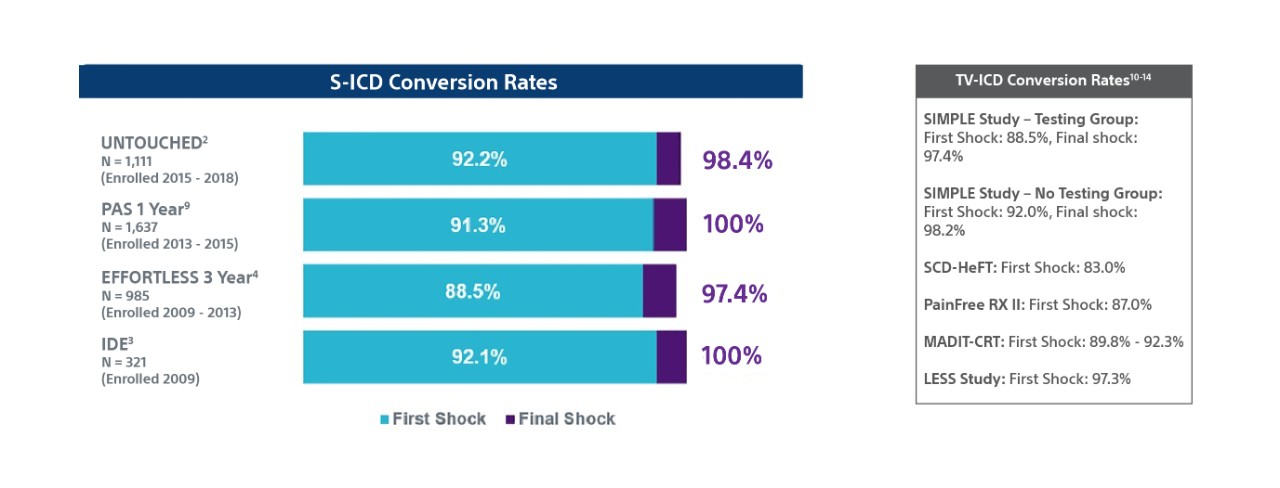
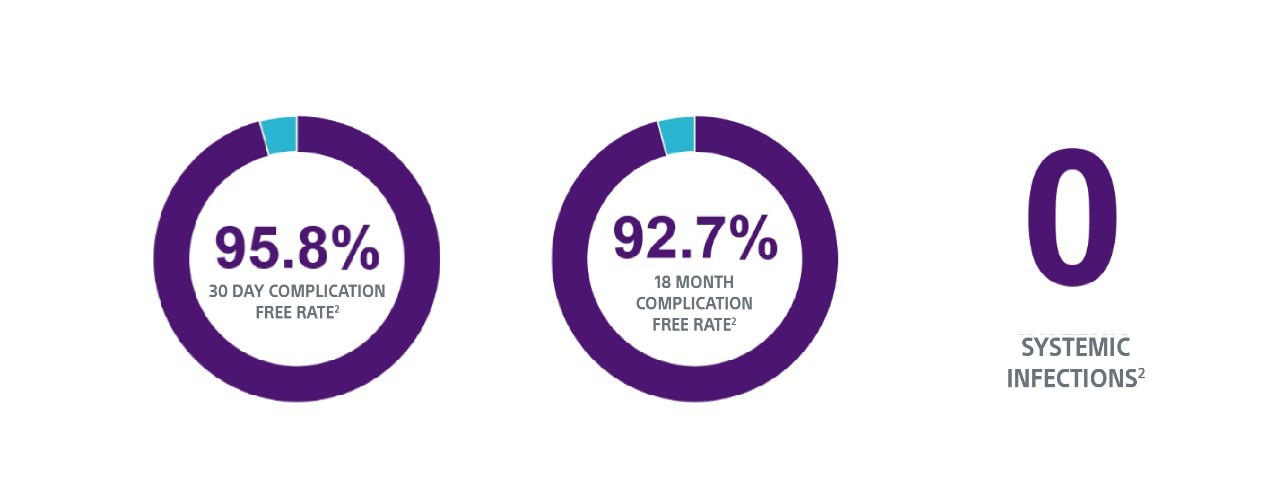
TV-ICD lead complications in the real world



Clinical data resources
The Impact of SMART Pass on IAS
Training & education

Explore continuing education courses, best practices modules and other training and resources for S-ICD.
Why S-ICD?
See how S-ICD helps protect patients at risk for sudden cardiac death while also eliminating the risk of TV-ICD lead complications.
