Our focus
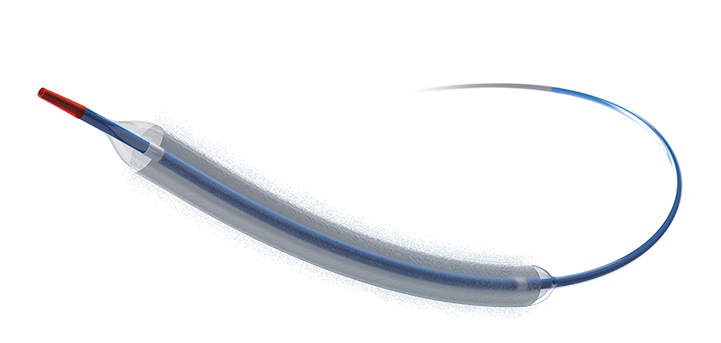
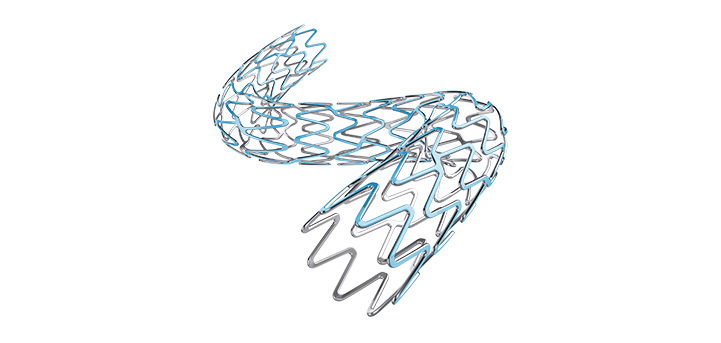
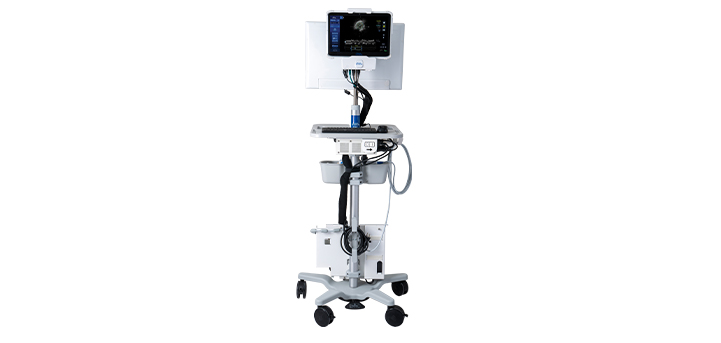
Minimally invasive technologies to improve the lives of patients living with heart and vascular conditions.
Jim’s story
We’re committed to advancing interventional cardiology therapies through less invasive technologies that improve outcomes, deliver care to more patients, reduce costs, and help millions live healthier lives.