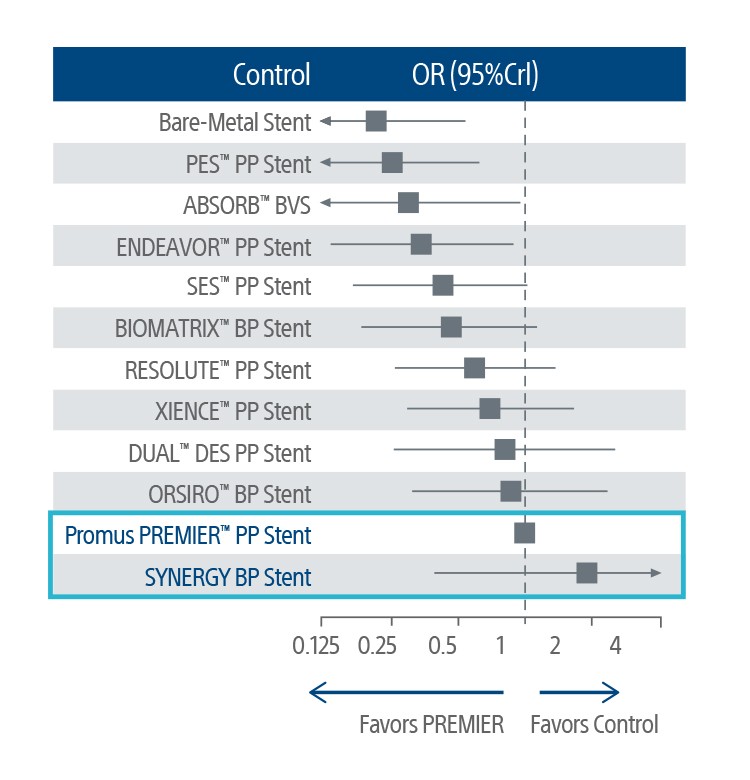
† Caution should be taken in interpreting study results, as some stents had limited numbers of comparisons, and some of the studies had a potential risk of bias. All PtCr-EES stents, also includes PROMUS ElementTM and PROMUS Element PlusTM. Def/prob ST was available in 110 studies with 111,088 patients.
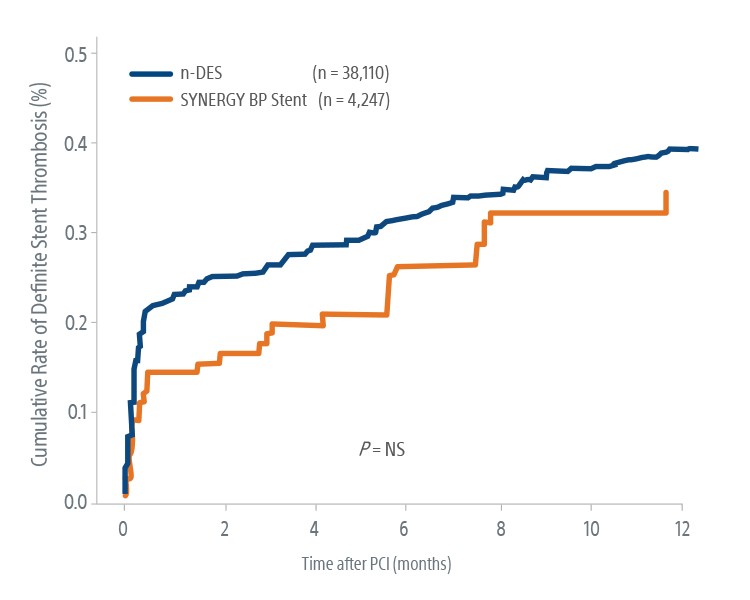
‡ Some patients in the SCAAR study were treated for either Left Main, CTO or multi-vessel disease but did not show statistical significance. The safety and efficacy of the SYNERGY BP Stent for those uses has not been established. A total of 7,886 of Synergy stents and 64,429 other n-DES (BioMatrix, N51,953; Orsiro, N54,946; Promus Element Plus, N5 2,543; Promus Premier, N5 20,414; Xience Xpedition, N5 7,971, Resolute/Resolute Integrity, N519,021; UltimasterTM, N51,156; Resolute Onyx, N56,425) were implanted in 42,357 procedures.
§ Cumulative adjusted ARC def ST estimated from Kaplan Meier Curve
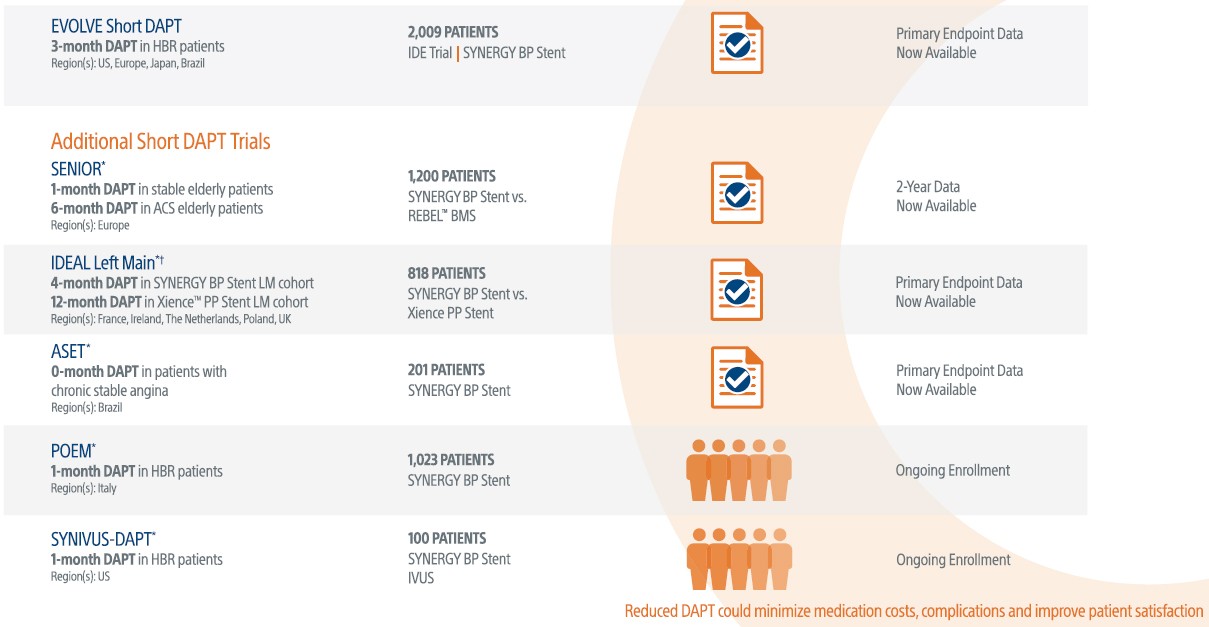
Supporting well-constructed prospective Short DAPT clinical trials with over 5,000 patients to study the SYNERGY™ Stent in various complex patient populations
Studies the safety of discontinuing DAPT at 3-months in high bleeding risk patients using the SYNERGY™ Stent.
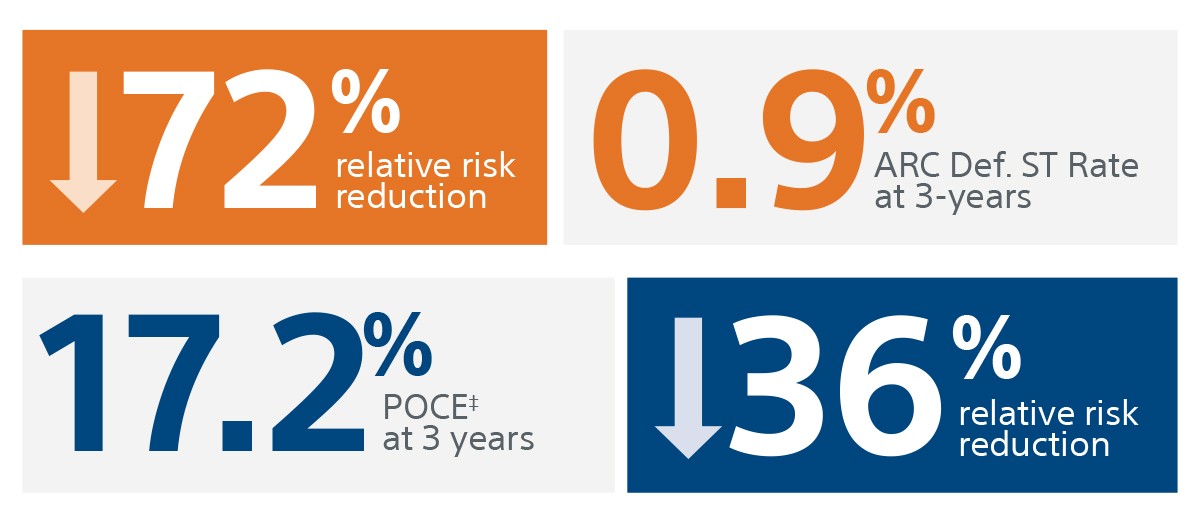
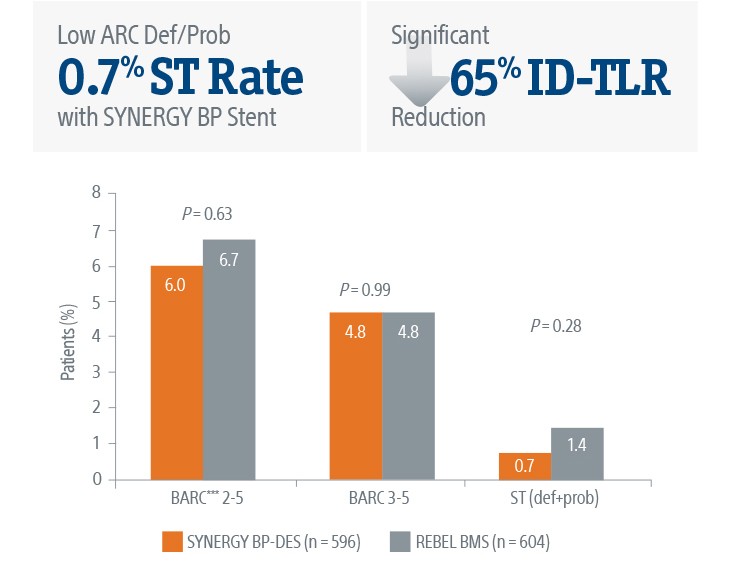
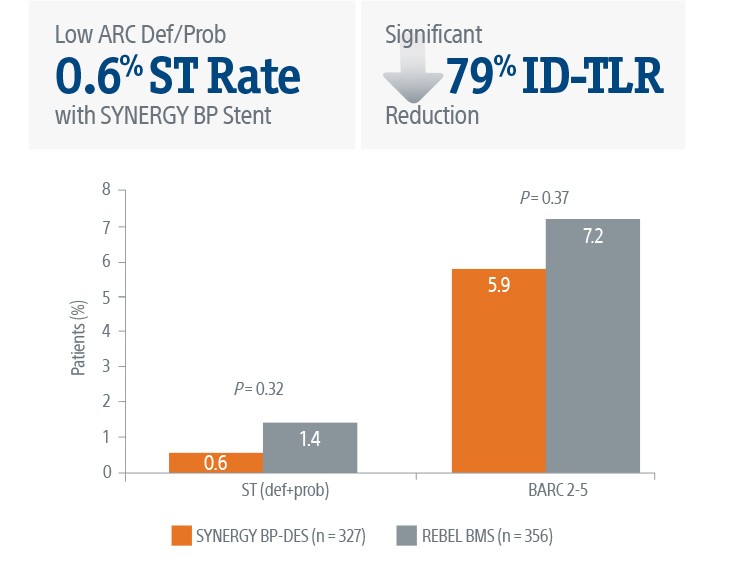
The SYNERGY™ BP Stent continued to show superior ID-TLR and safety results versus REBEL™ BMS with short BMS-like DAPT regimen at 2-Years.
Studying 0-Month DAPT in Patients with Chronic Stable Angina.
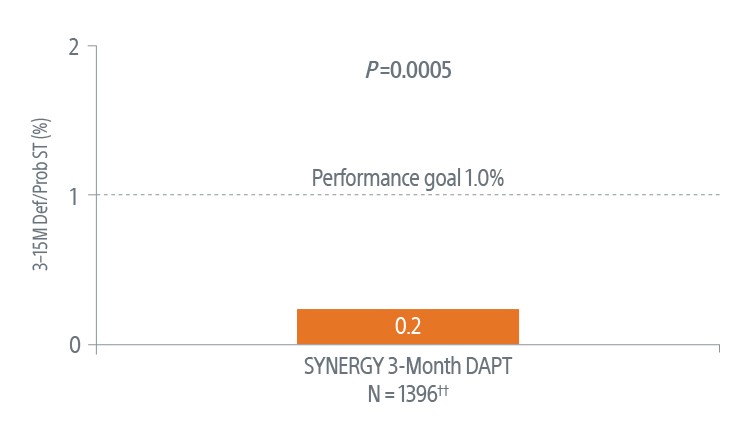
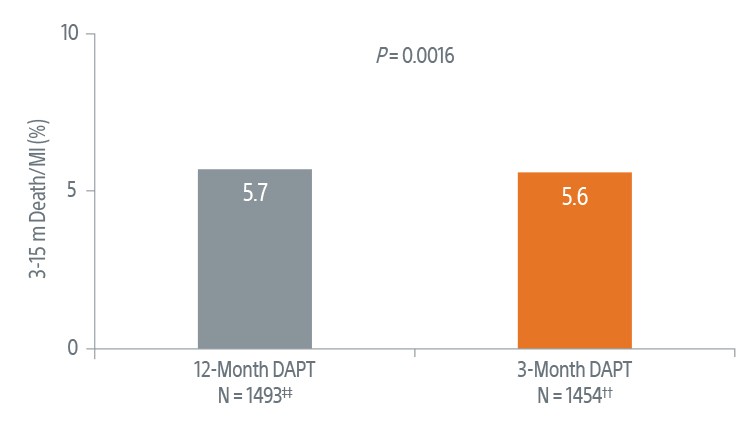
Safety Data at 3-Months4
Prasugrel monotherapy immediately after SYNERGY™ BP Stent implantation demonstrated encouraging safety in selected patients with low ischemic and bleeding risk.
Zero Definite ST
with the SYNERGY™ BP Stent in
selected patients with low risk stable CAD
ASET is a multicenter, single arm, proof-of-concept trial evauating the feasibility and safety of a single anti platelet therapy with prasugrel, starting immediately after successful PCI with the SYNERGY™ BP Stent (i.e. no DAPT; aspirin-free monotherapy).
EVOLVE 48 Trial: 1-year results
Supports the safety and effectiveness of the SYNERGY™ 48 mm BP Stent for the treatment of long lesions.†††7
- 100 patients
- 15 sites across US, Europe, and New Zealand
- 100% B2/C lesions
- 27% diabetes mellitus
††† Clinical data conducted with SYNERGY 48 mm, SYNERGY XD’s predecessor device, which can be used to illustrate SYNERGY XD 48 mm clinical data.
‡‡‡ Defined as any ischemia-driven target lesion revascularization, target vessel-related myocardial infarction, or cardiac death
§ Cumulative adjusted ARC def ST estimated from Kaplan Meier Curve
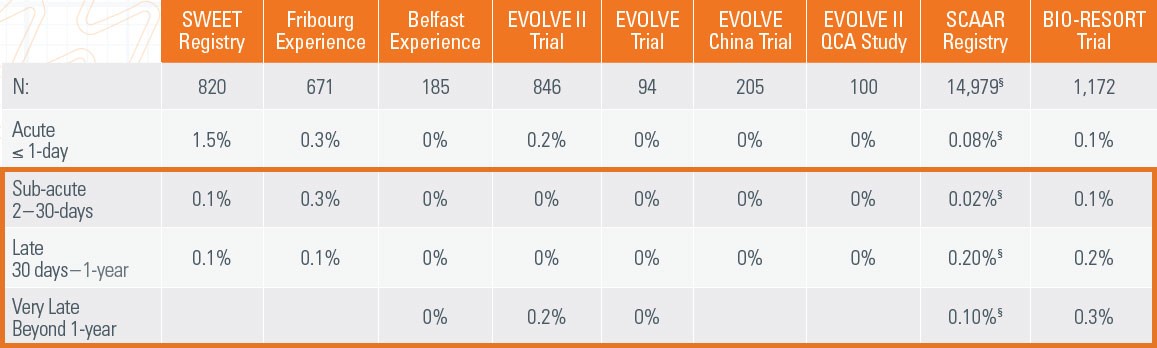
17. SWEET Registry: Cook TCT 2015., Fribourg Experience: Arroyo CRT 2016. Belfast Experience: Noad TCT 2015., EVOLVE II: Kereiakes, et al. Circ Cardiovasc
Interv. 2015;8:e002372. doi:10.1161/CIRCINTERVENTIONS.114.002372.,EVOLVE FHU: Meredith et al. J Am Coll Cardiol. 2012;59(15):1362., EVOLVE II QCA: Meredith ACC 2015., SCAAR Registry: James TCT 2016 BIO-RESORT presented by Clemens von Birgelen, MD, PhD, TCT 2016. BIORESORT: , Marlies M. Kok et al. Euro Interv.:2018;14-online publish-ahead-of-print May 2018.