Medical Specialties > Structural Heart > SENTINEL™ Cerebral Protection System > SENTINEL™ Results
A protected TAVI case
Watch as Professor Bernard Prendergast and Professor Simon Redwood from St. Thomas Hospital, London protect an intermediate risk TAVI patient from the potential risk of stroke.
Physician perspectives on embolic debris during TAVI
"Whenever you implant a valve debris will dislodge and travel to the brain."
Dr. N. Van Mieghem – Erasmus MC, The Netherlands

Seeing is believing
In 99% of procedures, SENTINEL was shown to capture and remove stroke-causing embolic debris.1

SENTINEL leads the way in evidence for cerebral embolic protection.
SENTINEL leads the way in clinical evidence for cerebral embolic protection for over 2,600 patients across a randomized trial and multiple registries.


1. SENTINEL IDE Trial. Data presented at SENTINEL FDA Advisory Panel, Feb 23, 2017.; 2. Van Mieghem N., TVT 2018 (includes TIA); 3. Stripe, B. PCR LV 2019; 4. Rinaldi, TCT 2018. 5. Chakravarty T., TCT 2018; 6. Seeger J., et al., JACC Cardiovasc Interv. 2017.
Fisher exact test; Results from different studies are not directly comparable. Information provided for educational purposes only.
New data show low risk patients are at similar stroke risk from embolic debris during TAVI
With TAVI moving to younger & lower risk patients is important to examine and analyze embolic debris as a potential risk of stroke. The Sentinel Low-Intermediate Registry provide some data on this.
The SENTINEL Low – Intermediate Registry:
Characterization of cerebral emboli capture using SENTINEL during TAVI1
STUDY DESIGN: N = 50 low-intermediate surgical-risk patients; Real-world, multicenter, prospective registry.
Clinical Highlights
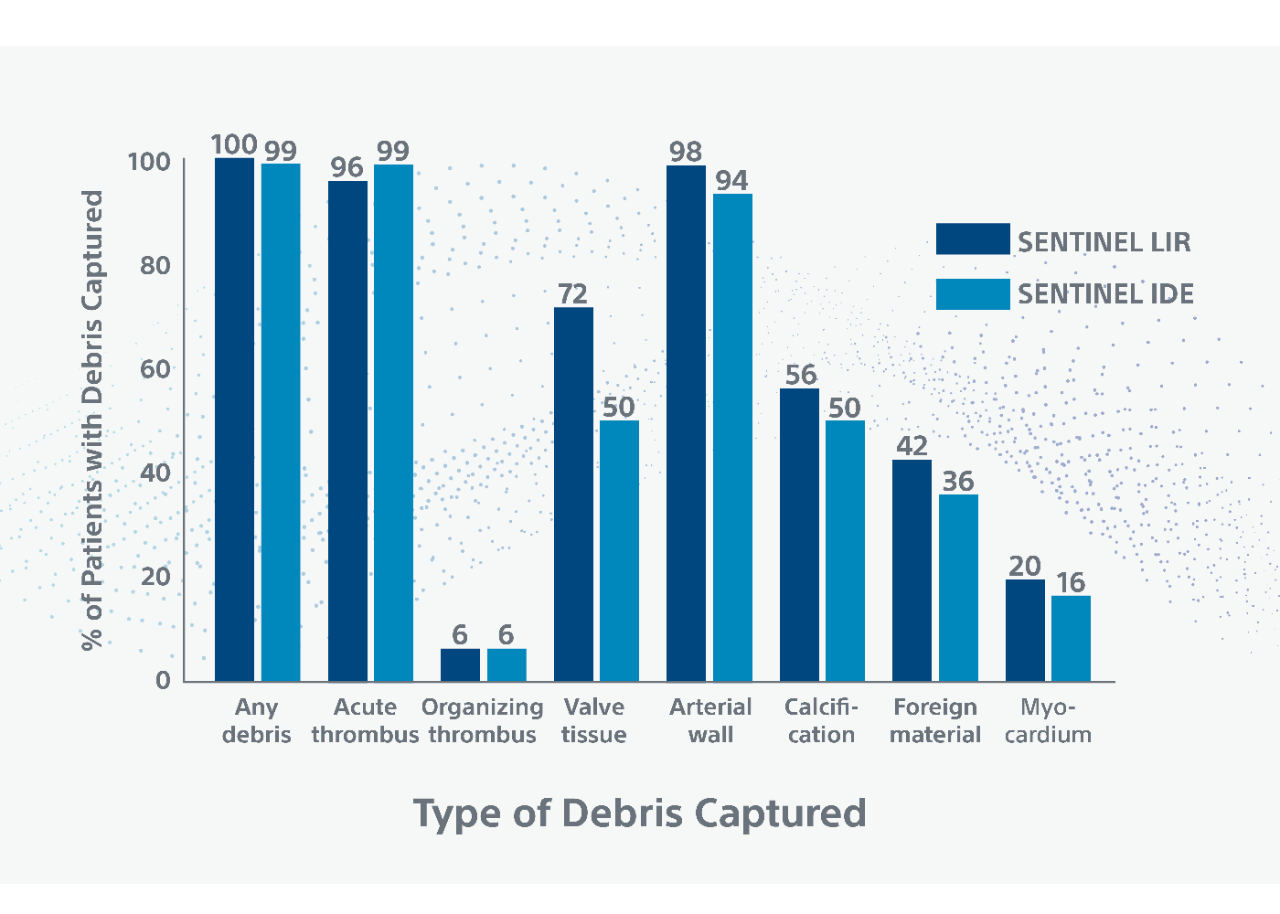
- Of 100 filters analyzed from 50 patients, debris was found in 100% (50/50) of patients*
- Debris type and size from low to intermediate surgical risk TAVI patients consistent in rate, type and particle size of captured debris in high surgical risk patients in the SENTINEL IDE Trial2
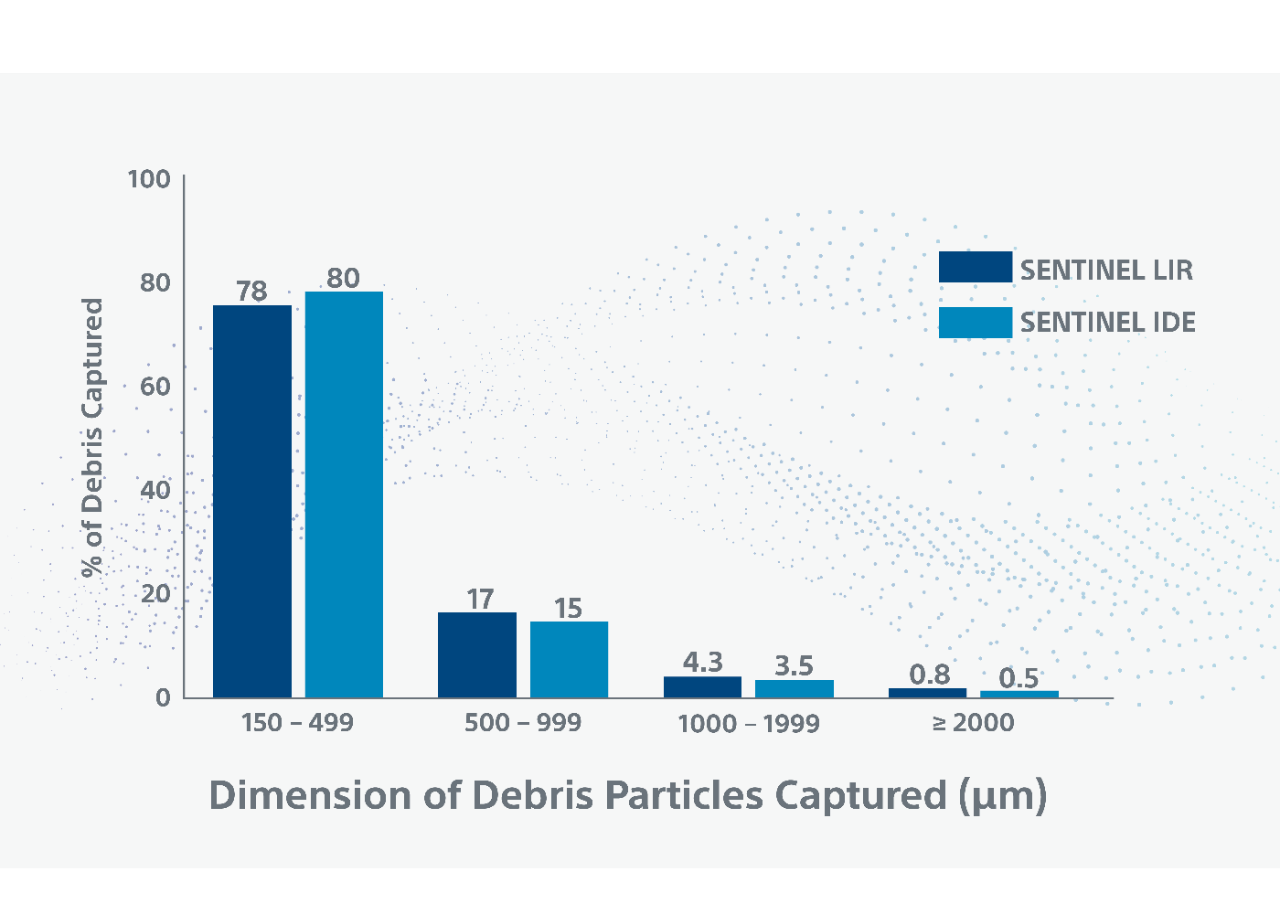
** Debris included arterial wall, acute thrombus, valve tissue, calcification and foreign material. Myocardium, organizing thrombus and necrotic core were observed less frequently. Majority (78%) of debris particles were 150-499 in maximum diameter; Larger size particles (≥1000), which can cause significant vessel obstruction, were present in 5% of patients.
1. The SENTINEL Low-Intermediate - Registry (LIR): Characterization of Cerebral Emboli Capture Using the Sentinel Device during TAVR in Low to Intermediate Risk Patients. Presented at CRT 2021 by Dr. Aloke Finn, CV Path Institute.
2. SENTINEL IDE Trial. Data presented at SENTINEL FDA Advisory Panel, February 23, 2017 .