SpaceOAR Vue™ System Perirectal Spacer for Prostate Radiation Therapy
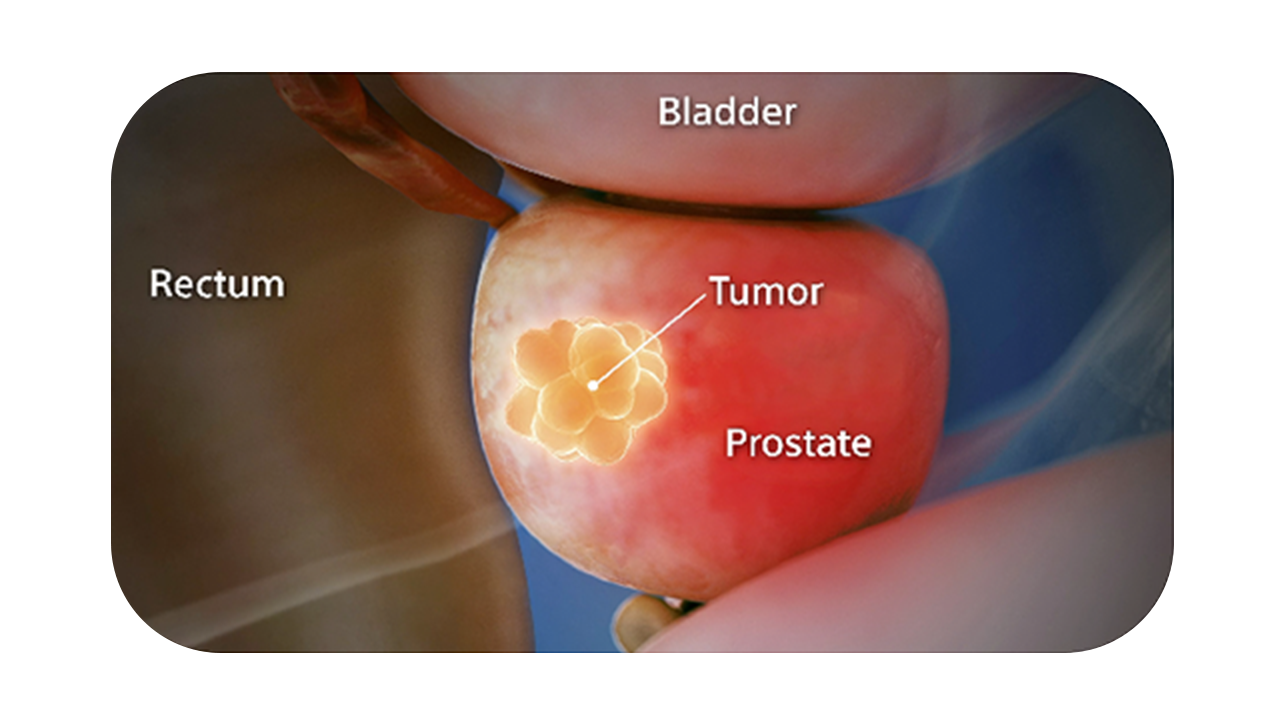
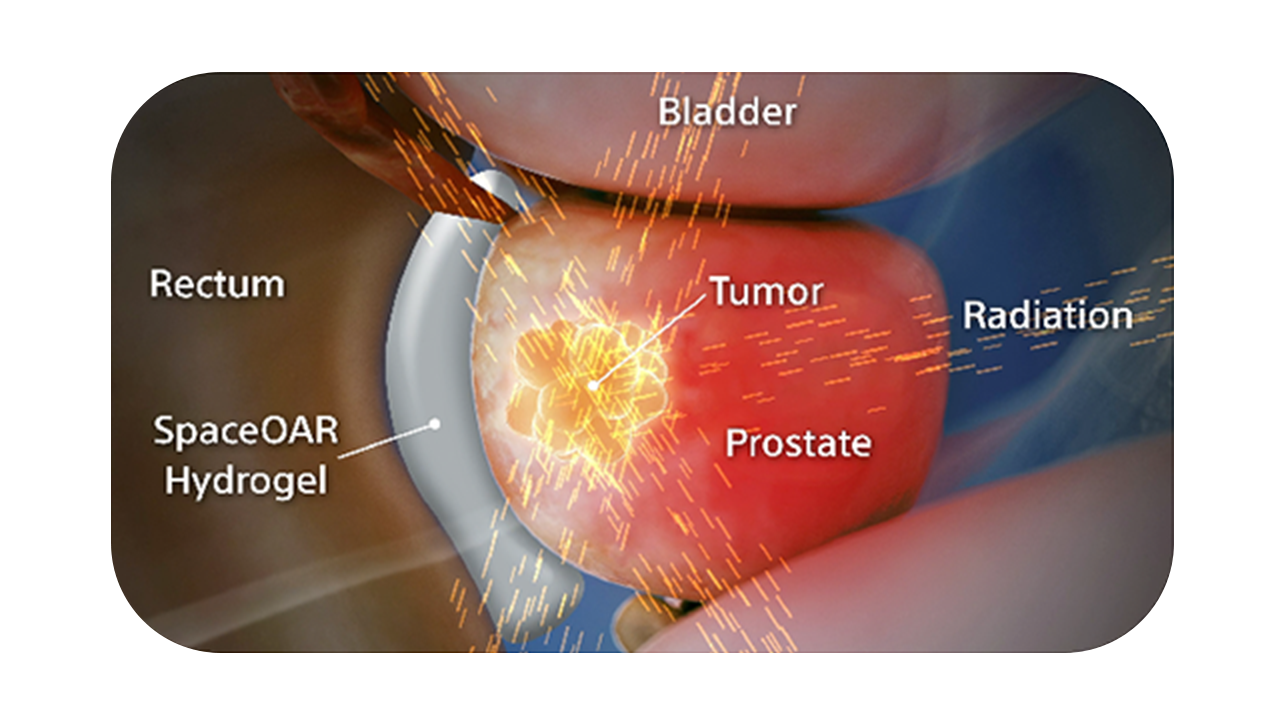
SpaceOAR Vue System is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR Vue System to reduce the radiation dose delivered to the anterior rectum.¹
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Ordering information
- Training
- Resources
You see it when you CT it.
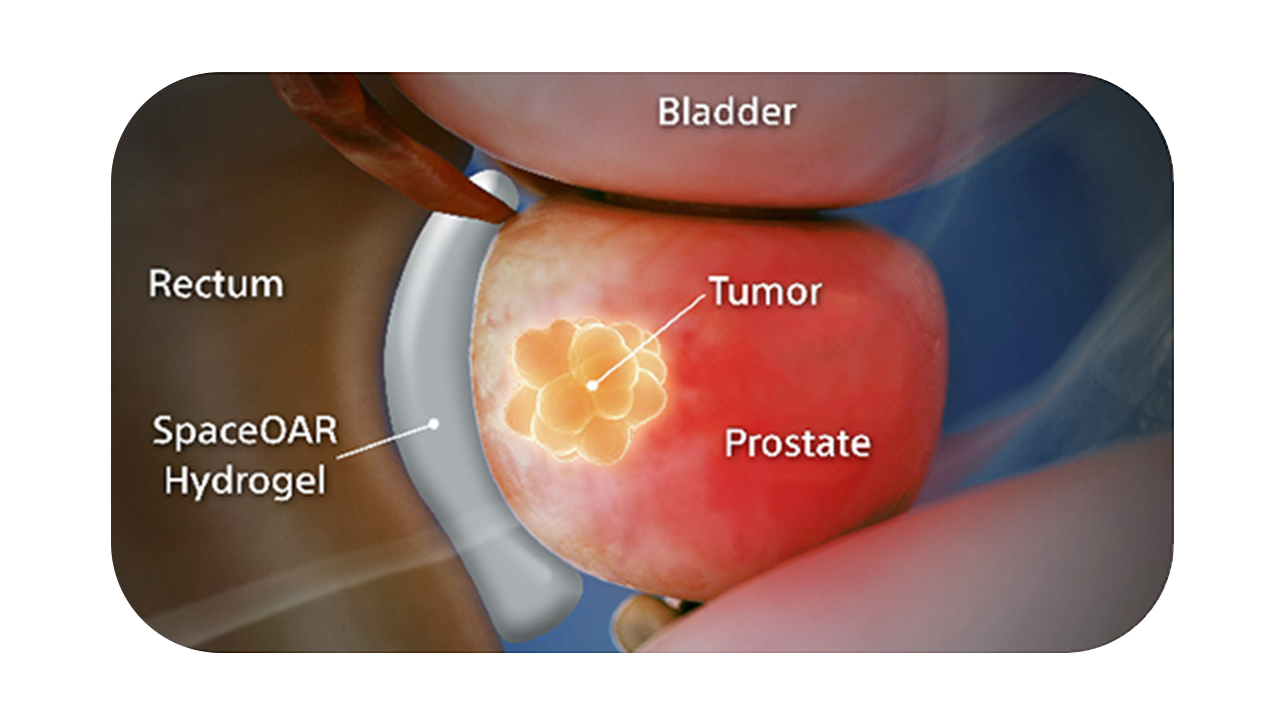
SpaceOAR Vue Hydrogel is the only FDA-cleared radiopaque PEG-based perirectal hydrogel spacer designed for CT visibility to help optimize radiotherapy treatment planning for prostate cancer.1
How it works
SpaceOAR Vue Hydrogel, the next-generation hydrogel perirectal spacer is designed to offer enhanced visibility on CT. Due to the close proximity between the rectum and the prostate, radiation therapy can unintentionally cause rectal toxicity. SpaceOAR Vue Hydrogel is designed to help reduce the radiation delivered to the rectum during treatment.1
Why choose SpaceOAR Vue Hydrogel
The radiopacity is designed to improve the contouring accuracy during treatment plan creation, when compared to SpaceOAR Hydrogel.1
Integrating SpaceOAR Vue Hydrogel into your practice
Boston Scientific supports a thorough in-service training program for office staff, clinical staff, and physicians in the practice. A Boston Scientific representative brings the training to you, and it can be completed in approximately 1 hour for each group.
Clinical data
SpaceOAR Vue Hydrogel: The Next-Generation Radiopaque Hydrogel Spacer
Clinical summary: “Summarized findings of adding the potential for CT visibility to SpaceOAR Vue Hydrogel is anticipated to expand hydrogel accessibility for patients who cannot undergo MRI, eliminate dependence on MRI for hydrogel contouring, assist in daily IGRT, and further improve the convenience of hydrogel placement.”
SpaceOAR Vue™ Hydrogel: The Next-Generation Radiopaque Hydrogel Spacer
Randall J. Brenneman, M.D., Ph.D., and Brian C. Baumann, M.D., Department of Radiation Oncology, Washington University School of Medicine, St. Louis, Missouri, United States
Ordering information
| Product | Ordering number |
| SpaceOAR System | SO-2101 |
| SpaceOAR System multi-pack, 4 units | SO-2104SB |
Medical education and physician training
Certified applier program
Our formal three-step certified applier program teaches physicians the proper SpaceOAR Hydrogel System application technique to enhance patient outcomes.
Complete these steps to become a certified applier
Enhance your technique with additional training
Peer-to-peer training
Opportunities include lectures, preceptorships, proctorships, and peer-to-peer calls.
Centers of education
Live case observation and discussions at one of our established centers of education.
Webinars
Targeted topics led by top healthcare experts.
National courses
Procedural and clinical education offered in partnership with UroGPO and The Urology Center of Colorado.
Resident in-services
Residents can train to participate in some, or all, of the procedure under the guidance of a certified applier.
Continuing nursing education
Intended primarily for RNs with responsibility for or interest in prostate diagnosis and treatment. Participants will be granted 1 contact hour CNE credit.
Simulator
Simulation training models a real-world SpaceOAR Hydrogel application.
Clinical education
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal and more accessible.
Register to access a library of procedural videos, case studies, training resources, and events.
Peer-to-peer education
Browse our full on-demand catalog of videos and editorials — many narrated and authored by practicing urologists — to stay up to date on the latest research on perirectal spacers for prostate cancer radiation therapy.
Product brochure
Learn how the polyethylene glycol (PEG) based hydrogel is designed to temporarily create space between the prostate and rectum.
Professional resources
Learn about our extensive education resources designed for you, including tools and resources focused on perirectal spacers for prostate cancer radiation therapy.
Patient resources
*SpaceOAR Vue is Hydrogel visible on CT when compared to non-radiopaque perirectal spacers
Reference
- Data on file with Boston Scientific.
SpaceOAR Vue Hydrogel is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR Vue Hydrogel to reduce the radiation dose delivered to the anterior rectum.
SpaceOAR Vue Hydrogel contains polyethylene glycol (PEG) and iodine.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, and potential adverse events.
As with any medical treatment, there are some risks involved with the use of SpaceOAR Vue Hydrogel. Potential complications associated with SpaceOAR Vue Hydrogel include, but are not limited to: pain associated with SpaceOAR Vue Hydrogel injection, pain or discomfort associated with SpaceOAR Vue Hydrogel, local inflammatory reactions, infection (including abscess), urinary retention, urgency, constipation (acute, chronic, or secondary to outlet perforation), rectal tenesmus/muscle spasm, mucosal damage, ulcers, fistula, perforation (including prostate, bladder, urethra, rectum), necrosis, allergic reaction (localized or more severe reaction, such as anaphylaxis), embolism (venous or arterial embolism is possible and may present outside of the pelvis, potentially impacting vital organs or extremities), syncope, and bleeding. The occurrence of one or more of these complications may require treatment or surgical intervention. URO-989810-AB
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.