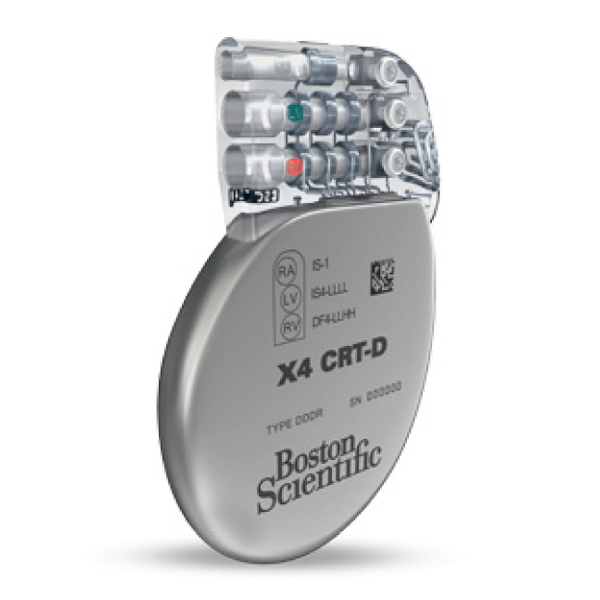
INOGEN™ X4
Cardiac Resynchronization Therapy Defibrillator (CRT-D)
INOGEN X4 features 17 pacing vectors and EnduraLifeTM Battery Technology.
Key Resources
Product Details
Models G146 and G148
- The INOGEN X4 CRT-D features 17 pacing vectors designed to provide more options for phrenic nerve stimulation avoidance and pacing threshold management.
- Features EnduraLife Battery Technology.
- Includes the new EasyView™ header with color coded lead ports, designed to improve implant efficiency.
- The world's thinnest high-energy CRT-D on the market.
Offers an uncompromised set of features
- An advanced system solution for patient comorbidities and HF monitoring (HF Perspectiv™ report, LATITUDE™ NXT Remote Patient Management* with weight scale and blood pressure sensors, and Respiratory Rate Trend).
- AcuShock™Advanced Technology, multiple programmable options to reduce inappropriate and unnecessary shocks, including a choice of rhythm discriminators, antitachycardia pacing (ATP) therapy in all rates zones, and advanced sensing and filtering.
- SafetyCore™ technology intended to provide lifesaving shock therapy and basic pacing functionality in the event of an unrecoverable fault.
*Caution: Investigational device. Limited by United States Federal Law to investigational use. Not for sale/use in the United States.
Mechanical Specifications
| Model | Type | Size (cm) (W x H x D) | Mass (g) | Volume (cc) | Connector Type (RA RV LV) |
|---|---|---|---|---|---|
| G148 | X4 CRT-D | 5.37 x 8.18 x 0.99 | 73.8 | 32.5 | RA:IS-1; RV:DF4; LV:IS4 |
| G146 | X4 CRT-D | 5.37 x 8.08 x 0.99 | 73.4 | 32.0 | RA:IS-1; RV:IS-1/DF-1; LV:IS4 |
Pacing Amplitude | Projected Longevity (years) at 500Ω and 700Ω Pacing Impedance (RV and LV) | ||
| RA/RV | LV | 500Ω | 700Ω |
| 2.5 V | 3.0 V | 8.1 | 8.6 |
| 2.5 V | 3.5 V | 7.6 | 8.2 |
| 3.5 V | 3.5 V | 6.8 | 7.5 |
| 3.5 V | 5.0 V | 5.7 | 6.5 |
a Assumes ZIP™ telemetry use for 3 hours at implant time and for 40 minutes annually for in-clinic follow-up checks.
b Assumes standard use of the LATITUDE™ Communicator as follows: Daily Device Check on, monthly Full Interrogations (scheduled remote follow ups, and quarterly patient-interrogations).
c Assumes 60 bpm LRL; DDDR mode; 100% biventricular pacing; 15% atrium pacing and 0.4 ms pacing Pulse Width (RA, RV, LV); RA Impedance 500 Ω; sensors On.
d Projected longevity is calculated assuming 3 maximum energy charging cycles per year, including automatic capacitor re-forms and therapeutic shocks. For the final year of device service, an additional 5 charging cycles are assumed to account for additional automatic capacitor re-forms as the device approaches the Explant indicator. These calculations also assume 3-channel EGM Onset is set to On, and that the pulse generator spends 6 months in Storage mode during shipping and storage.
Longevity Information
- For longevity calculations based on different settings please contact Boston Scientific technical services or your local representative.
- Boston Scientific devices have a limited product warranty of 6 years in available geographies.
- Devices use Li/MnO2 battery chemistry.
- The usable battery capacity is 1.9 Amp-hours (typical implant to battery capacity depleted).
- Shelf life is 2 years (before use by date).