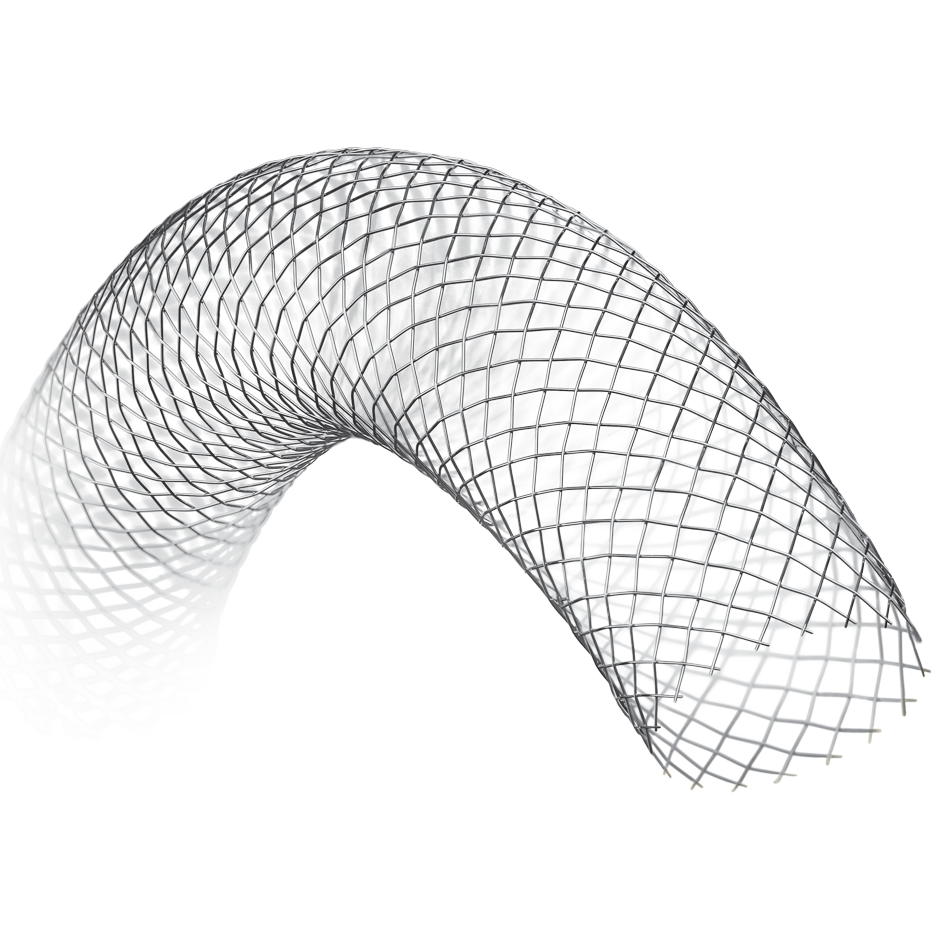
WALLSTENT™
Endoprosthesis
Designed for ease of placement, the WALLSTENT Endoprosthesis allows reconstrainability with the stent deployed up to the limit marker band.
Key Resources
Product Detail
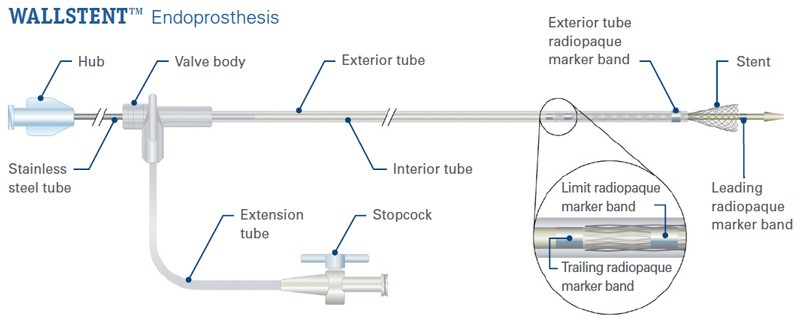
Ordering Information
WALLSTENT™ Endoprosthesis
| UPN/Order Code | Catalog No. | GTIN | Unconstrained Diameter* (mm) | Unconstrained Length* (mm) | Guide Sheath Compatibility (F) | Guide Catheter Compatibility |
|---|---|---|---|---|---|---|
| M001719000 | 71-900 | 6 | 22 | 5 | 7 | |
| M001719010 | 71-901 | 8 | 21 | 5 | 7 | |
| M001719020 | 71-902 | 8 | 29 | 5 | 7 | |
| M001719030 | 71-903 | 8 | 36 | 5 | 7 | |
| M001719040 | 71-904 | 10 | 24 | 6 | 8 | |
| M001719050 | 71-905 | 10 | 31 | 6 | 8 | |
| M001719060 | 71-906 | 10 | 37 | 6 | 8 |
The C-code used for this product is C1876, Stent, non-coated/non-covered with delivery system. C-codes are used for hospital outpatient device reporting for Medicare and some private payers.
Note: Boston Scientific is not responsible for the correct use of codes on submitted claims; this information does not constitute reimbursement or legal advice.
Tools and Resources
-
 Aug 24, 2017
Aug 24, 2017MRI Compatibility for PI Products
Magnetic Resonance Imaging (MRI) Safety for Boston Scientific Peripheral Products PDF, 272.0 KB
-