Express™ SD
Renal and Biliary Premounted Stent System
The Express SD Stent couples excellent compression resistance with lower stent foreshortening and recoil. Only Express SD has the ideal balance of strength and precision for acute success.
Key Resources
Product Details
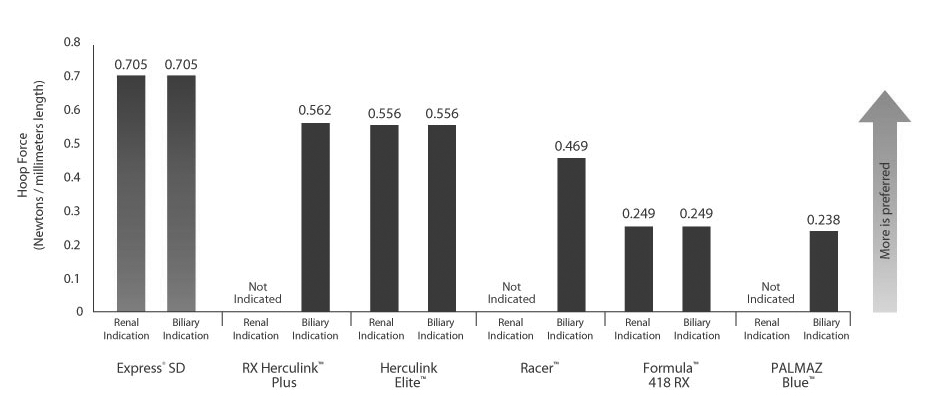
Best-in-Class Compression Resistance
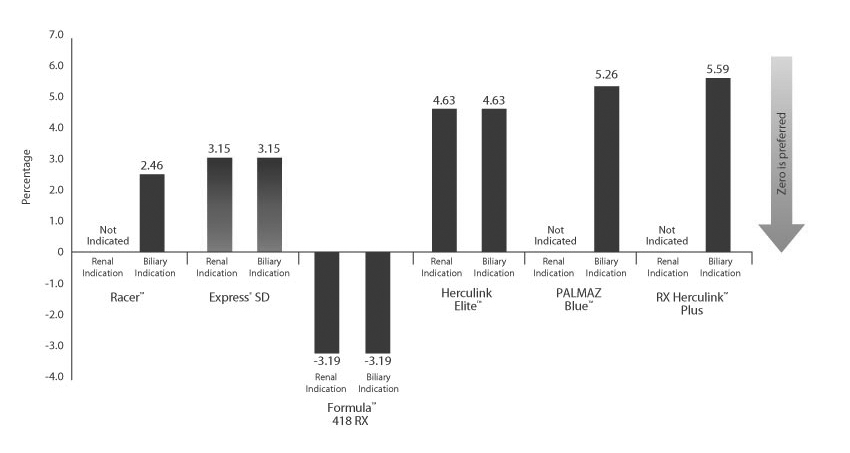
Minimal Foreshortening
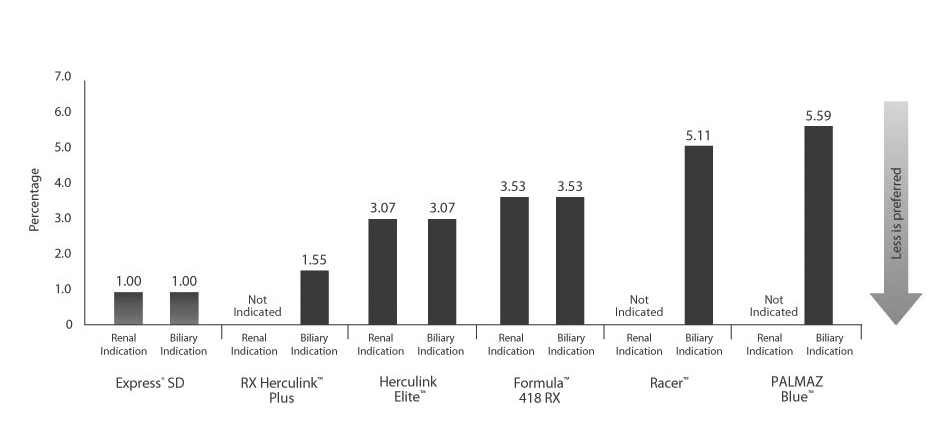
Very Low Recoil
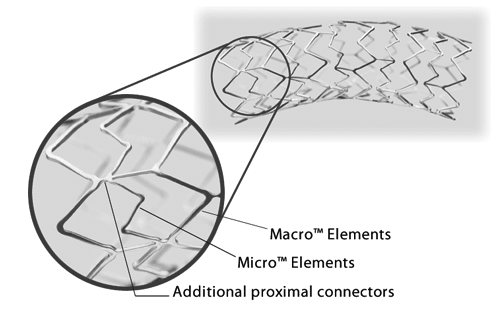
Express SD features a patented Tandem Architecture™ Stent Design
- Macro™ Elements and additional proximal connectors engineered to provide strength, especially in ostial lesions
- Micro™ Elements designed to provide flexibility during stent placement
Clinical Information
RENAISSANCE Clinical Trial
Overview The RENAISSANCE Trial was a prospective, multi-center, single arm study designed to evaluate the Express SD Renal Premounted Stent System in the treatment of atherosclerotic lesions in the aortorenal ostium.
The primary objective was to demonstrate superiority of the duplex triggered, angiographically-confirmed binary restenosis rate at 9-months over the 40% pre-specified Objective Performance Criterion (OPC) representative of PTRA.
Trial Design The RENAISSANCE Trial enrolled 100 patients (117 lesions) with de novo or restenotic ostial atherosclerotic lesions ≤15mm long in vessels between 4.0 and 7.0mm diameter with diameter stenosis ≥70%.
Outcomes Primary Endpoint: 21.3% binary in-stent restenosis rate at 9-months, defined as the proportion of target lesions with ≥50% diameter stenosis based on angiographic core lab assessment.
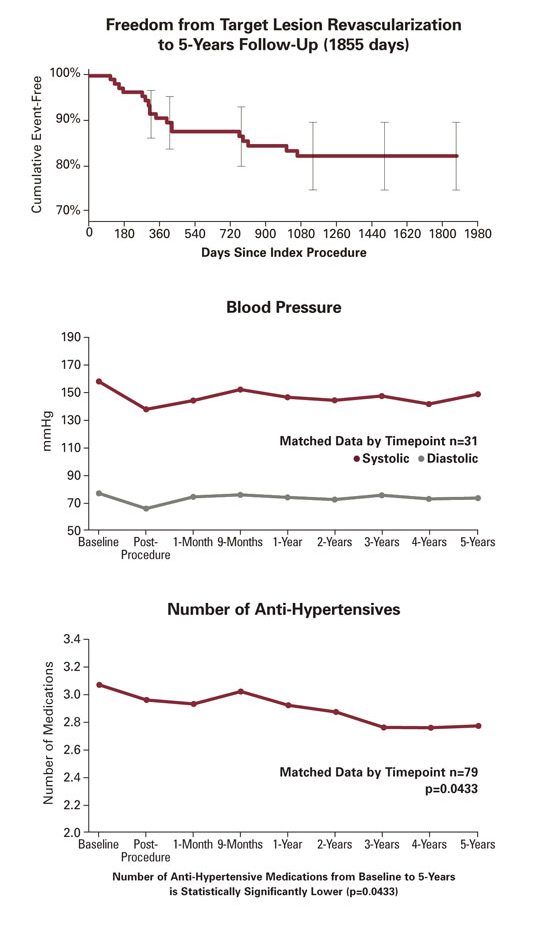
5-Year Data
Additional Results
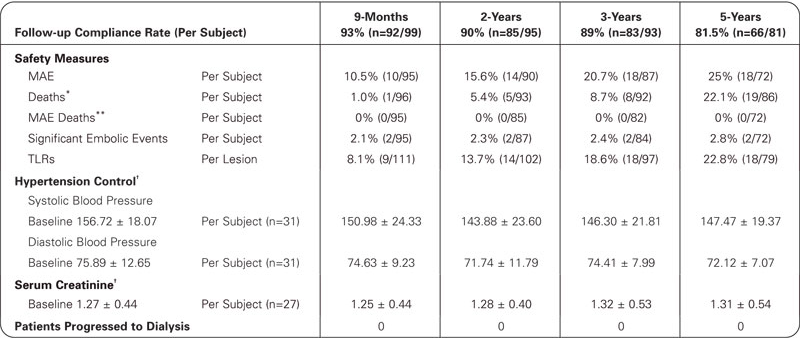
*Denominator consists of number of subjects who either died prior to late follow-up window and/or had follow-up to at least the early follow-up window.
**Denominator consists of number of subjects who either had CEC adjudicated device or procedure related death prior to late follow-up window and/or had follow-up to at least the early follow-up window.
†Matched data by time point.
For additional information, please see Rocha-Singh K, Jaff M, Kelley E.L. Renal Artery Stenting with Non-Invasive Duplex Ultrasound Follow-up: 3-Year Results from the RENAISSANCE Renal Stent Trial. Catheterization and Cardiovascular Interventions 2008; 72:853-862.
Tools & Resources
-
 Aug 24, 2017
Aug 24, 2017MRI Compatibility for PI Products
Magnetic Resonance Imaging (MRI) Safety for Boston Scientific Peripheral Products PDF, 272.0 KB
-